Two objects with different temperatures can exchange
energy, if they are in thermal contact.
The energy exchanged between object because they are in thermal contact
is called heat. If two objects are
in thermal contact and do not exchange heat, then they are in
thermal equilibrium.
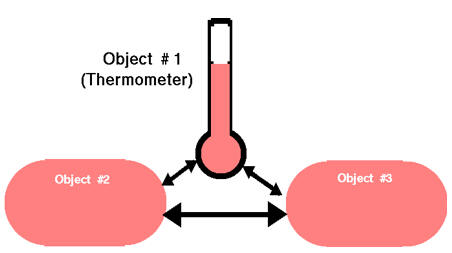
The zeroth law of thermodynamics states that two object,
which are separately in thermal equilibrium with a third object, are in thermal
equilibrium with each other.
Two objects in thermal equilibrium with each
other are at the same temperature.
How can we heat things up?
- We can add thermal energy to an object by doing work on the
object. If we rub an object, the force of sliding friction
does work and changes ordered kinetic energy into thermal energy.
- We can burn something. If fuel burns, chemical energy
is converted into thermal energy.
 Atoms in molecules and solids are held together by
chemical bonds. Chemical bonds are electromagnetic in origin, but
can be modeled well by tiny springs. Two atoms held together by a
spring have an equilibrium position. If they are pushed closer
together, they repel each other. If they are pulled farther apart,
they attract each other. If they are displaced in any way from
their equilibrium position and then released, they start vibrating
about their equilibrium position. An atom can form different
chemical bonds with a variety of other atoms. Different bonds are
represented by springs with different spring constants. The stiffer
the spring, the more work it takes to pull the atoms apart. If
enough work is done, then the spring is stretched too much and it
breaks, i.e. the chemical bond breaks.
Atoms in molecules and solids are held together by
chemical bonds. Chemical bonds are electromagnetic in origin, but
can be modeled well by tiny springs. Two atoms held together by a
spring have an equilibrium position. If they are pushed closer
together, they repel each other. If they are pulled farther apart,
they attract each other. If they are displaced in any way from
their equilibrium position and then released, they start vibrating
about their equilibrium position. An atom can form different
chemical bonds with a variety of other atoms. Different bonds are
represented by springs with different spring constants. The stiffer
the spring, the more work it takes to pull the atoms apart. If
enough work is done, then the spring is stretched too much and it
breaks, i.e. the chemical bond breaks.
At room temperature, gas molecules have
random translational kinetic energy associated with the motion of
their center of mass and random vibrational energy and rotational
kinetic energy associated with the motion about their center of
mass. Collisions continuously transfer energy between the
different degrees of freedom and the average energy in each degree
of freedom is the same. If work is done on the molecules which
increases their vibrational energy, the amplitude of the vibrations
increases, and eventually the chemical bonds break. Most free
atoms quickly form new bonds. If the new bonds are
stronger, i.e. if the new springs are stiffer, then they do more
work pulling the atom towards their new equilibrium positions than
was needed to break the old bonds, and the
atoms will have more kinetic energy as they pass through the
equilibrium positions. This kinetic energy is quickly
shared with the other degrees of freedom, the energy of all degrees
of freedom increases, i.e. the thermal energy increases. Thermal energy is released by a
chemical reaction.
The temperature increases.
To burn fuel, work must first be done to break the chemical bonds
in the fuel. This work provides the
activation energy, the energy
needed to start the chemical reaction. The free atoms and
molecules then bond with oxygen. The new bonds with the oxygen
atoms are much stronger than the broken bonds.
As the atoms form new bonds, they gain thermal energy.
When you strike a match, you first do work against friction to break
the chemical bonds in some of the fuel on the head. The free
atoms and molecules now combine with oxygen from the air, forming
stronger bonds and thus releasing thermal energy. The random kinetic energy of these
fast molecules is transferred in collisions to neighboring atoms and
molecules, breaking their bonds, etc.
Heat flow
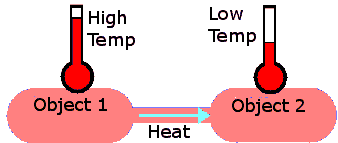 When you bring two objects of different temperature
together, energy will always be transferred from the hotter to the
cooler object. The objects will exchange thermal energy, until thermal equilibrium is reached, i.e. until their temperatures are equal. We say that
heat flows from the hotter to the cooler object. Heat is
energy on the move.
When you bring two objects of different temperature
together, energy will always be transferred from the hotter to the
cooler object. The objects will exchange thermal energy, until thermal equilibrium is reached, i.e. until their temperatures are equal. We say that
heat flows from the hotter to the cooler object. Heat is
energy on the move.
Units of heat are units of energy. The SI unit of energy is
Joule. Other often encountered units of energy are 1 Cal = 1 kcal
= 4186 J, 1 cal = 4.186 J, 1 Btu = 1054 J.
Without an external agent doing work, heat will always flow from a
hotter to a cooler object. Two objects of different temperature always
interact. There are three different ways for heat to flow from one
object to another. They are conduction,
convection, and
radiation.
Conduction
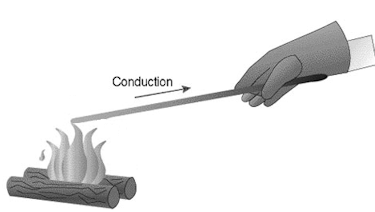 The atoms in a solid vibrate about their equilibrium positions. As
they vibrate, they bump into their neighbors. In those collisions they
exchange energy with their neighbors. If the different regions of a
solid object or of several solid objects placed in contact with each
other have the same temperature, then all atoms are just as likely to
gain energy as to loose energy in the collisions. Their average random
kinetic energy does not change. If, however, one region has a higher
temperature than another region, then the atoms in the high temperature
region will, on average, loose energy in the collisions, and the atoms
in the low temperature region will, on average, gain energy. In this
way heat flows through a solid by conduction.
The atoms in a solid vibrate about their equilibrium positions. As
they vibrate, they bump into their neighbors. In those collisions they
exchange energy with their neighbors. If the different regions of a
solid object or of several solid objects placed in contact with each
other have the same temperature, then all atoms are just as likely to
gain energy as to loose energy in the collisions. Their average random
kinetic energy does not change. If, however, one region has a higher
temperature than another region, then the atoms in the high temperature
region will, on average, loose energy in the collisions, and the atoms
in the low temperature region will, on average, gain energy. In this
way heat flows through a solid by conduction.
The stiffness of the springs (strength of the chemical bonds)
determines how easily the atoms can exchange energy and therefore
determines if the material is a good or bad conductor of heat. Each
atom has a nucleus, surrounded by electrons. In a solid metal all
nuclei are bound to their equilibrium positions. But some electrons are
free to move throughout the solid. They can easily pick up kinetic
energy in collisions with hot cores and loose it again in collision with
cooler cores. Since their mean free path between collisions is
larger than the distance between neighboring atoms, thermal energy can
move quickly through the material. Metals are, in
general, much better conductors of heat than insulators.
Convection
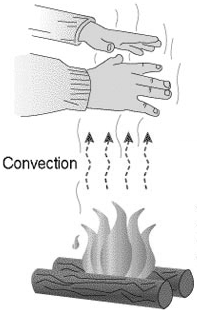 Convection transfers heat via the motion of a fluid which contains
thermal energy. In an environment where a constant gravitational force
F = mg acts on every object of mass m, convection develops
naturally because of changes in the fluid's density with temperature.
When a fluid, such as air or water, is in contact with a hotter object,
it picks up thermal energy by conduction. Its density decreases. For a
given volume of the fluid, the upward buoyant force equals the weight of
this volume of cool fluid. The downward force is the weight of this
volume of hot fluid. The upward force has a larger magnitude than the
downward force and the volume of hot fluid rises. Similarly, when a
fluid is in contact with a colder object, it cools and sinks. When a
volume of fluid such as air or water starts to move, the surrounding
fluid has to rush in to fill the void. Otherwise large pressure
differences would develop. This sets up a convection current and the looping path that follows is a
convection cell. Since fluid cannot pile up at some point in space without creating a high-pressure
area, it will flow in a closed loop. Convection can be increased if the
fluid is forced to circulate. A fan, for example, forces the air to
circulate.
Convection transfers heat via the motion of a fluid which contains
thermal energy. In an environment where a constant gravitational force
F = mg acts on every object of mass m, convection develops
naturally because of changes in the fluid's density with temperature.
When a fluid, such as air or water, is in contact with a hotter object,
it picks up thermal energy by conduction. Its density decreases. For a
given volume of the fluid, the upward buoyant force equals the weight of
this volume of cool fluid. The downward force is the weight of this
volume of hot fluid. The upward force has a larger magnitude than the
downward force and the volume of hot fluid rises. Similarly, when a
fluid is in contact with a colder object, it cools and sinks. When a
volume of fluid such as air or water starts to move, the surrounding
fluid has to rush in to fill the void. Otherwise large pressure
differences would develop. This sets up a convection current and the looping path that follows is a
convection cell. Since fluid cannot pile up at some point in space without creating a high-pressure
area, it will flow in a closed loop. Convection can be increased if the
fluid is forced to circulate. A fan, for example, forces the air to
circulate.
Video: Convection Current (Youtube)
Radiation
 Nuclei and electrons are charged particles. When charged particles
accelerate, they emit electromagnetic radiation
and loose energy. Vibrating particles are always accelerating since
their velocity is always changing. They therefore always emit
electromagnetic radiation. Charged particles also absorb
electromagnetic radiation. When they absorb the radiation they
accelerate. Their random kinetic energy increases. In thermal
equilibrium, the amount of energy they lose to radiation equals the
amount of energy they gain from radiation. But hotter objects emit more
radiation than they absorb from their cooler environment. Radiation can
therefore transport heat from a hotter to a cooler object.
Nuclei and electrons are charged particles. When charged particles
accelerate, they emit electromagnetic radiation
and loose energy. Vibrating particles are always accelerating since
their velocity is always changing. They therefore always emit
electromagnetic radiation. Charged particles also absorb
electromagnetic radiation. When they absorb the radiation they
accelerate. Their random kinetic energy increases. In thermal
equilibrium, the amount of energy they lose to radiation equals the
amount of energy they gain from radiation. But hotter objects emit more
radiation than they absorb from their cooler environment. Radiation can
therefore transport heat from a hotter to a cooler object.
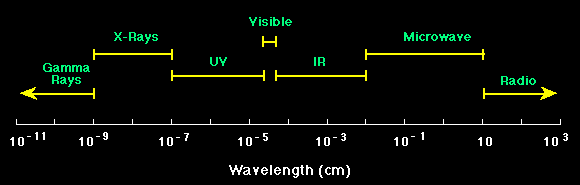
Electromagnetic radiation refers to
electromagnetic waves, which travel through free space with the
speed of light. We classify electromagnetic waves according to their
wavelength. A graphical representation of the electromagnetic spectrum
is shown in the figure below.
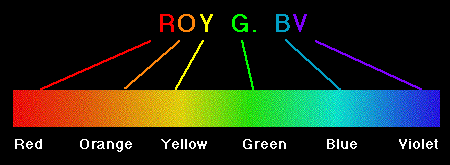
The visible part of the spectrum may be
further subdivided according to color, with red at the long wavelength
end and violet at the short wavelength end, as illustrated in the next
figure.
 Hot objects emit radiation with a distribution of wavelengths. But
the average wavelength of the radiation decreases as the temperature of
the object increases. Most thermal radiation lies in the infrared
region of the spectrum. We cannot see this radiation, but we can feel
it warming our skin. Different objects emit and absorb infrared
radiation at different rates. Dark surfaces are generally good
emitters.
Hot objects emit radiation with a distribution of wavelengths. But
the average wavelength of the radiation decreases as the temperature of
the object increases. Most thermal radiation lies in the infrared
region of the spectrum. We cannot see this radiation, but we can feel
it warming our skin. Different objects emit and absorb infrared
radiation at different rates. Dark surfaces are generally good
emitters.
Examples of all heat transfer processes:
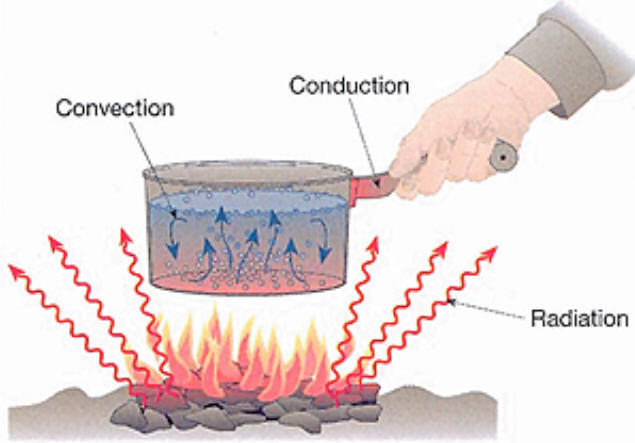
When a wood stove is used to heat the air in a room, conduction,
convection, and radiation play a role.
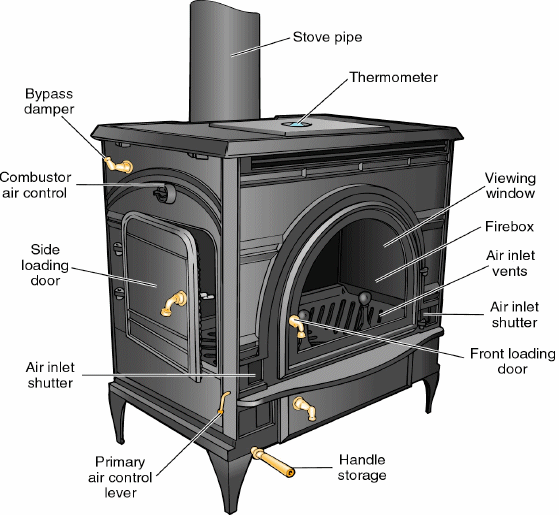
When the wood burns, chemical energy stored in the wood is converted
into thermal energy of the reaction products. By conduction,
these reaction products heat the surfaces and the air they are in
contact with.
 Convection draws the hot smoke up a long black pipe and out of
the room and draws fresh air into the stove. When the smoke is in
contact with inner the surface of the pipe, it heats the pipe by
conduction. Conduction also carries the thermal energy from
the inner surfaces of the stove and the pipe to the outer surfaces, and
heats the air close to the surfaces. The hot air then begins to rise by
convection. Cooler air rushes in to replace the rising air, and
a convection current begins to flow in a convection cell.
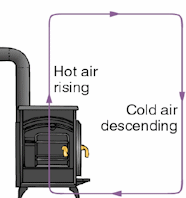
Convection draws the hot smoke up a long black pipe and out of
the room and draws fresh air into the stove. When the smoke is in
contact with inner the surface of the pipe, it heats the pipe by
conduction. Conduction also carries the thermal energy from
the inner surfaces of the stove and the pipe to the outer surfaces, and
heats the air close to the surfaces. The hot air then begins to rise by
convection. Cooler air rushes in to replace the rising air, and
a convection current begins to flow in a convection cell.
This distributes the warm air throughout the room. The hot, black,
outer surface of the stove is also a good emitter of infrared thermal
radiation. This thermal radiation is absorbed by the surfaces of
different objects in the room.
 Atoms in molecules and solids are held together by
chemical bonds. Chemical bonds are electromagnetic in origin, but
can be modeled well by tiny springs. Two atoms held together by a
spring have an equilibrium position. If they are pushed closer
together, they repel each other. If they are pulled farther apart,
they attract each other. If they are displaced in any way from
their equilibrium position and then released, they start vibrating
about their equilibrium position. An atom can form different
chemical bonds with a variety of other atoms. Different bonds are
represented by springs with different spring constants. The stiffer
the spring, the more work it takes to pull the atoms apart. If
enough work is done, then the spring is stretched too much and it
breaks, i.e. the chemical bond breaks.
Atoms in molecules and solids are held together by
chemical bonds. Chemical bonds are electromagnetic in origin, but
can be modeled well by tiny springs. Two atoms held together by a
spring have an equilibrium position. If they are pushed closer
together, they repel each other. If they are pulled farther apart,
they attract each other. If they are displaced in any way from
their equilibrium position and then released, they start vibrating
about their equilibrium position. An atom can form different
chemical bonds with a variety of other atoms. Different bonds are
represented by springs with different spring constants. The stiffer
the spring, the more work it takes to pull the atoms apart. If
enough work is done, then the spring is stretched too much and it
breaks, i.e. the chemical bond breaks.
 When you bring two objects of different temperature
together, energy will always be transferred from the hotter to the
cooler object. The objects will exchange thermal energy, until thermal equilibrium is reached, i.e. until their temperatures are equal. We say that
heat flows from the hotter to the cooler object. Heat is
energy on the move.
When you bring two objects of different temperature
together, energy will always be transferred from the hotter to the
cooler object. The objects will exchange thermal energy, until thermal equilibrium is reached, i.e. until their temperatures are equal. We say that
heat flows from the hotter to the cooler object. Heat is
energy on the move. The atoms in a solid vibrate about their equilibrium positions. As
they vibrate, they bump into their neighbors. In those collisions they
exchange energy with their neighbors. If the different regions of a
solid object or of several solid objects placed in contact with each
other have the same temperature, then all atoms are just as likely to
gain energy as to loose energy in the collisions. Their average random
kinetic energy does not change. If, however, one region has a higher
temperature than another region, then the atoms in the high temperature
region will, on average, loose energy in the collisions, and the atoms
in the low temperature region will, on average, gain energy. In this
way heat flows through a solid by conduction.
The atoms in a solid vibrate about their equilibrium positions. As
they vibrate, they bump into their neighbors. In those collisions they
exchange energy with their neighbors. If the different regions of a
solid object or of several solid objects placed in contact with each
other have the same temperature, then all atoms are just as likely to
gain energy as to loose energy in the collisions. Their average random
kinetic energy does not change. If, however, one region has a higher
temperature than another region, then the atoms in the high temperature
region will, on average, loose energy in the collisions, and the atoms
in the low temperature region will, on average, gain energy. In this
way heat flows through a solid by conduction. Convection transfers heat via the motion of a fluid which contains
thermal energy. In an environment where a constant gravitational force
F = mg acts on every object of mass m, convection develops
naturally because of changes in the fluid's density with temperature.
When a fluid, such as air or water, is in contact with a hotter object,
it picks up thermal energy by conduction. Its density decreases. For a
given volume of the fluid, the upward buoyant force equals the weight of
this volume of cool fluid. The downward force is the weight of this
volume of hot fluid. The upward force has a larger magnitude than the
downward force and the volume of hot fluid rises. Similarly, when a
fluid is in contact with a colder object, it cools and sinks. When a
volume of fluid such as air or water starts to move, the surrounding
fluid has to rush in to fill the void. Otherwise large pressure
differences would develop. This sets up a convection current and the looping path that follows is a
convection cell. Since fluid cannot pile up at some point in space without creating a high-pressure
area, it will flow in a closed loop. Convection can be increased if the
fluid is forced to circulate. A fan, for example, forces the air to
circulate.
Convection transfers heat via the motion of a fluid which contains
thermal energy. In an environment where a constant gravitational force
F = mg acts on every object of mass m, convection develops
naturally because of changes in the fluid's density with temperature.
When a fluid, such as air or water, is in contact with a hotter object,
it picks up thermal energy by conduction. Its density decreases. For a
given volume of the fluid, the upward buoyant force equals the weight of
this volume of cool fluid. The downward force is the weight of this
volume of hot fluid. The upward force has a larger magnitude than the
downward force and the volume of hot fluid rises. Similarly, when a
fluid is in contact with a colder object, it cools and sinks. When a
volume of fluid such as air or water starts to move, the surrounding
fluid has to rush in to fill the void. Otherwise large pressure
differences would develop. This sets up a convection current and the looping path that follows is a
convection cell. Since fluid cannot pile up at some point in space without creating a high-pressure
area, it will flow in a closed loop. Convection can be increased if the
fluid is forced to circulate. A fan, for example, forces the air to
circulate. Nuclei and electrons are charged particles. When charged particles
accelerate, they emit electromagnetic radiation
and loose energy. Vibrating particles are always accelerating since
their velocity is always changing. They therefore always emit
electromagnetic radiation. Charged particles also absorb
electromagnetic radiation. When they absorb the radiation they
accelerate. Their random kinetic energy increases. In thermal
equilibrium, the amount of energy they lose to radiation equals the
amount of energy they gain from radiation. But hotter objects emit more
radiation than they absorb from their cooler environment. Radiation can
therefore transport heat from a hotter to a cooler object.
Nuclei and electrons are charged particles. When charged particles
accelerate, they emit electromagnetic radiation
and loose energy. Vibrating particles are always accelerating since
their velocity is always changing. They therefore always emit
electromagnetic radiation. Charged particles also absorb
electromagnetic radiation. When they absorb the radiation they
accelerate. Their random kinetic energy increases. In thermal
equilibrium, the amount of energy they lose to radiation equals the
amount of energy they gain from radiation. But hotter objects emit more
radiation than they absorb from their cooler environment. Radiation can
therefore transport heat from a hotter to a cooler object.

 Hot objects emit radiation with a distribution of wavelengths. But
the average wavelength of the radiation decreases as the temperature of
the object increases. Most thermal radiation lies in the infrared
region of the spectrum. We cannot see this radiation, but we can feel
it warming our skin. Different objects emit and absorb infrared
radiation at different rates. Dark surfaces are generally good
emitters.
Hot objects emit radiation with a distribution of wavelengths. But
the average wavelength of the radiation decreases as the temperature of
the object increases. Most thermal radiation lies in the infrared
region of the spectrum. We cannot see this radiation, but we can feel
it warming our skin. Different objects emit and absorb infrared
radiation at different rates. Dark surfaces are generally good
emitters.

 Convection draws the hot smoke up a long black pipe and out of
the room and draws fresh air into the stove. When the smoke is in
contact with inner the surface of the pipe, it heats the pipe by
conduction. Conduction also carries the thermal energy from
the inner surfaces of the stove and the pipe to the outer surfaces, and
heats the air close to the surfaces. The hot air then begins to rise by
convection. Cooler air rushes in to replace the rising air, and
a convection current begins to flow in a convection cell.
Convection draws the hot smoke up a long black pipe and out of
the room and draws fresh air into the stove. When the smoke is in
contact with inner the surface of the pipe, it heats the pipe by
conduction. Conduction also carries the thermal energy from
the inner surfaces of the stove and the pipe to the outer surfaces, and
heats the air close to the surfaces. The hot air then begins to rise by
convection. Cooler air rushes in to replace the rising air, and
a convection current begins to flow in a convection cell.