PV = NkBT is called the ideal gas law. It
is the equation of state, which relates the quantities V, P, and
T. Most real gases at ordinary temperatures and
pressures obey the ideal gas law. The ideal gas law can be rewritten as
PV = nNAkBT = nRT.
Here n is the number of moles of the gaseous substance, NA
is Avogadro's number, NA = 6.022*1023
molecules/mol, and R = NAkB is a constant, called
the universal gas constant, R = 8.31 J/(mol K).
The number of moles n is given by n = m/M where m is the average mass of the gas
particles in the volume, and M is the molar mass of the gas.
In the 17th and 18th century experiment with gases at
very low temperature and pressures revealed three relations that are generalized
by the ideal gas law.
- For a low-density gas at constant temperature, P is inversely proportional
to V.
Boyle's law: PV = constant (at constant T).
- For a low-density gas at constant volume the pressure is proportional to
the temperature.
Law of Gay-Lussac: P = constant * T
(at constant V).
- For a low-density gas at constant pressure the volume is proportional to
the temperature.
Charles's Law: V = constant * T (at constant P).
The ideal gas law holds well for real gases at low
densities and pressures, such as atmospheric density and pressure. If we use T
= 0 oC = 273 K and P = 1 atm, then we find that one mole of gas
occupies a volume of 22.4 liters. One mole of gas contains NA gas
particles. For all low density gases, NA gas particles occupy
22.4 liters at T = 273K and P = 1 atm.
Nothing in the ideal gas law depends on the nature of the gas particles. The
value of PV/T depends only on the number of gas particles and a universal
constant.
Note: In all gas laws T denote the absolute temperature, measured in Kelvin
in SI units.
Problem:
The particle density of atmospheric air at 273.15 K at sea level is 2.687*1025/m3.
Calculate the pressure P.
Solution:
- Reasoning:
The ideal gas law, PV = NkBT, P = (N/V)kBT = ρparticlekBT.
- Details of the calculation:
P = (2.687*1025/m3)(1.381*10-23 Pa m3/K)(273.15
K) = 1.01*105 Pa = 101 kPa.
Problem:
If a helium-filled balloon initially at room temperature is placed in a freezer,
will its volume increase, decrease, or remain the same?
Solution:
- Reasoning:
The ideal gas law states that PV/T is constant. The pressure in the
freezer is atmospheric pressure, the temperature in the freezer is lower
that the outside temperature, so the volume of the balloon decreases when it
is placed into the freezer.
External link:
Balloons in Liquid
Nitrogen (Youtube)
Problem:
A tank having a volume of 0.1 m3 contains helium gas at 150 atm.
How many balloons can the tank blow up, if each filled balloon is a sphere 0.3 m
in diameter at an absolute pressure of 1.2 atm?
Solution:
- Reasoning:
At constant temperature P1V1 = P2V2.
(Boyle's law)
- Details of the calculation:
P1 = 150 atm = 1.515*107 Pa. V1
= 0.1 m3.
P2 = 1.2 atm = 1.212*105 Pa.
V2
= P1V1/P2 = 12.5 m3.
Let n = number of balloons and Vb
= the volume of each blown-up
balloon.
Vb = (4π/3)r3 = 1.414*10-2 m3.
n = V2/Vb
= 884. The tank can blow up 884 balloons.
Problem:
The mass of a hot air balloon and its cargo (not including the air inside) is
200 kg. The air outside is at 10 oC and 101 kPa. The volume of the
balloon is 400 m3. To what temperature must the air in the balloon
be heated before the balloon will lift off. (Air density at 10 oC is
1.25 kg/m3.)
Solution:
- Reasoning:
For the balloon to lift off, the buoyant force B must be greater than its
weight.
The buoyant force is equal to the weight of the
displaced air at 10 oC = 283 K.
B = (1.25 kg/m3)(400 m3)(9.8 m/s2)
= 4900 N.
The weight of the balloon is 200 kg(9.8 m/s2) + weight of hot
air.
The hot air therefore must weigh less than 4900 N - 1960 N = 2940 N.
Its mass must be less than 2940 N/(9.8 m/s2) = 300 kg.
Its density must be less than ρ = 300 kg/(400 m3) = 0.75 kg/m3.
At constant pressure, the volume of a gas is proportional to the absolute
temperature. (Law of Gay-Lussac) The pressures on the inside and outside
of the inflated balloon are nearly equal. The pressure on the outside is
the constant atmospheric pressure. The Law of Gay-Lussac therefore applies.
Since the volume of a gas at constant pressure is proportional to its
temperature, its density ρ = m/V is proportional to 1/T.
We have ρ1/ρ2
= T2/T1. ρ1T1/ρ2
= T2.
- Details of the calculation:
(1.25 kg/m3)(283 K)/(0.75 kg/m3) = 472 K = T2.
The air in the balloon must be heated to more than 472 K = 199 oC.
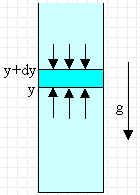 The earth atmosphere is a gas in the presence of gravity. Gravity prevents
the atmosphere from escaping. Near the Earth's surface the acceleration due to
gravity is approximately constant (g = 9.8 m/s2). The atmosphere is
a fluid, and the pressure in this fluid is a function of the altitude. For a
liquid, i.e. an incompressible fluid, we found that P = P0 + ρgy,
where y is the distance below the reference point. But the atmosphere is
a compressible gas. Let us consider a column of gas containing ρparticle
particles of mass m per unit volume near the Earth's surface.
The earth atmosphere is a gas in the presence of gravity. Gravity prevents
the atmosphere from escaping. Near the Earth's surface the acceleration due to
gravity is approximately constant (g = 9.8 m/s2). The atmosphere is
a fluid, and the pressure in this fluid is a function of the altitude. For a
liquid, i.e. an incompressible fluid, we found that P = P0 + ρgy,
where y is the distance below the reference point. But the atmosphere is
a compressible gas. Let us consider a column of gas containing ρparticle
particles of mass m per unit volume near the Earth's surface.
The pressure times the area (i.e. the force) at height y must exceed that at
height y + dy by the weight of the intervening gas. We need
Py+dyA - PyA = -mρparticleVg
= -mρparticlegAdy, or
dP = -mρparticlegdy.
But from the ideal gas law we know that P = ρparticlekT, with k
being the Boltzmann constant. We may therefore write
dP = -P(mg/kT)dy, or
dP/P = -(mg/kT)dy.
If the temperature T is constant, then this integrates to
P = P0e-mgy/(kT).
The pressure decreases exponentially with altitude.
Near Earth's surface the temperature T is not constant, it generally
decreases with altitude y. In the standard atmosphere T = T0 - ay,
with a = 0.0065 K/m. We then have
dP/P = -mgdy/(k(T0 - ay)).
This integrates to
ln(P/P0) =( mg/ka)ln(1 - ay/T0), or
(P/P0)(ka/mg) = (1 - ay/T0).
Since the Earth's atmosphere consists mostly of nitrogen molecules, we have
(P/P0)0.19 = (1 - (0.0065 (K/m))y/T0).
T0 and P0 are the temperature (in Kelvin) and the
pressure at sea level and the altitude y is measured in m. The pressure,
density, and temperature of the atmosphere decrease as the altitude increases.
External link: Standard
Atmosphere Calculator
How can we make a balloon rise?
We have to make its weight smaller than the weight of an equal shaped volume
of surrounding air. The skin of the balloon and the basket certainly are
heavier than an equal volume of the surrounding air. The inside of the skin
therefore has to be filled with something appreciably lighter than the
surrounding air. Ideally we would empty it out completely. But then the
pressure inside would be zero. The air pressure from the outside would collapse
the skin. To keep the skin stretched, the inside and outside pressures on each
section of skin must be equal, but the weight of the inside material must be
less than that of an equal volume of surrounding air. The pressure is
proportional to the particle density and the temperature, while the weight is
proportional to the particle density and the weight of each particle.
P = kρparticleT,
weight = ρparticle * volume * weight per particle.
To get the weight down and keep the pressure the same we have two options.
- Option 1:
We can decrease the weight per particle. Air is composed of
77% nitrogen, 21% oxygen and 2% other gases by weight. The density of air
at room temperature and atmospheric pressure is approximately 1.3 kg/m3.
If we replace the air with Helium gas at the same temperature, the same
particle density will yield the same pressure. But the mass density of
Helium gas at room temperature and atmospheric pressure is only
approximately 0.18 kg/m3. If we make the balloon big enough, the
total volume of air displaced by the balloon will weigh more than the
balloon itself, and the net force on the balloon will be upward. Since the
density of air decreases with altitude, the weight of the volume of air
displaced by the balloon will decrease as the Helium balloon rises. Eventually it will equal the weight of the
balloon and the net force on the balloon will be zero. As the balloon is
rising, it is also acted on by the drag force. The direction of the drag
force is opposite the direction of the balloon's velocity. Because the drag
force the the acceleration of the balloon will quickly decrease to close to
zero and the balloon will rise with nearly constant velocity. You can
explore this behavior in an exercise associated with this module.
- Option 2:
We can increase the temperature of the air in the balloon.
Then we can obtain the same pressure with a smaller particle density. Again
the weight of the balloon decreases and the net force on the balloon is
upward. The hot air balloon will rise.
 The earth atmosphere is a gas in the presence of gravity. Gravity prevents
the atmosphere from escaping. Near the Earth's surface the acceleration due to
gravity is approximately constant (g = 9.8 m/s2). The atmosphere is
a fluid, and the pressure in this fluid is a function of the altitude. For a
liquid, i.e. an incompressible fluid, we found that P = P0 + ρgy,
where y is the distance below the reference point. But the atmosphere is
a compressible gas. Let us consider a column of gas containing ρparticle
particles of mass m per unit volume near the Earth's surface.
The earth atmosphere is a gas in the presence of gravity. Gravity prevents
the atmosphere from escaping. Near the Earth's surface the acceleration due to
gravity is approximately constant (g = 9.8 m/s2). The atmosphere is
a fluid, and the pressure in this fluid is a function of the altitude. For a
liquid, i.e. an incompressible fluid, we found that P = P0 + ρgy,
where y is the distance below the reference point. But the atmosphere is
a compressible gas. Let us consider a column of gas containing ρparticle
particles of mass m per unit volume near the Earth's surface.