
Specific heat capacities:
(kcal/(kgoC))
| Water | 1.0 |
|---|---|
| Ice | 0.49 |
| Steam | 0.48 |
| Glass | 0.20 |
| Steel | 0.11 |
| Copper | 0.092 |
| Aluminum | 0.215 |
The unit kcal (kilocalorie) is a unit of energy.
1 kcal = 4186 J
In units of kcal/(kgoC) the specific heat of water is 1.
When heat flows from one material to another, the temperature of the contact layer of the cooler material increases. From the contact layer thermal energy has to spread throughout the cooler material by conduction or convection. How efficiently heat is transferred depends on the specific heat capacity of the material. The specific heat capacity c is the amount of energy it takes to raise the temperature of one kg of material by 1 degree Kelvin or Celsius.
c = ΔQ/(m ΔT).
Specific heat capacities: (kcal/(kgoC))
The specific heat capacity of water is approximately 4 times higher than that of air. The exact specific heat capacity of a substance depends on the condition under which it is measured. For gases, the specific heat capacity measured at constant volume is different from the specific heat capacity measured at constant pressure.
The smaller the specific heat capacity of a material that touches your skin, the less heat it takes to bring the temperature of the boundary layer up to the temperature of your skin. How fast the heat is carried away from this boundary layer now depends on the thermal conductivity of the material and on whether or not convection currents are present. To minimize heat loss from your skin, surround it by material of low specific heat capacity and low conductivity, and prevent convection. In addition you must minimize heat loss via radiation.
A 50 g sample of copper is at 25 oC. If 1200 J of thermal energy is added to it, what is the final temperature of the copper?
Solution:
An aluminum calorimeter of mass 100 g contains 250 g of water. The
calorimeter and water are in thermal equilibrium at 10 oC.
Two metallic blocks are placed in the water. One is a 50 g piece of
copper at 80oC. The other has a mass of 70 g and is
originally at a temperature of 100 oC. The entire system
stabilizes at a final temperature of 20 oC.
(a) Determine the specific heat of the unknown sample.
(b) Guess the material of the unknown sample.
Solution:
Why is the temperature of coastal cities fairly steady, but
in the desert it can vary considerably between night and day?
Answer:
Water is the key factor here. Water has a high specific heat so it is able to
retain, absorb and release a lot of energy. Near the coast, water absorbs heat
during the day and stores it, releasing it at night, acting like a heat sink.
In the desert there is little water to store energy and release it in such a
cycle, so the temperature is much more sensitive to the sun's being out or not.
Matter exists in different states. It can, for example, be in a solid state, a liquid state, or a gaseous state. These states are called phases. The atoms and molecules making up the matter move differently in different phases.



Solid
Liquid
Gas
In general, heat flows from an object of higher temperature to an object of lower temperature. Temperature is the quantity that indicates whether or not heat will flow and in what direction it will flow. When heat flows from a hotter to a cooler object, the temperature of the hotter object decreases, while the temperature of the cooler object rises. The average kinetic energy of the molecules in the hotter object decreases, while the average kinetic energy of the molecules in the cooler object increases. But when an object changes phase, its temperature does not change, even though heat is added or removed. The melting of ice and the boiling of water are familiar examples. During a change of phase the temperature does not change, but the internal energy does. The internal energy is the sum of the kinetic energy of the molecules and the chemical potential energy of the molecules. During a change of phase, the average kinetic energy of the molecules stays the same, but the average potential energy changes.
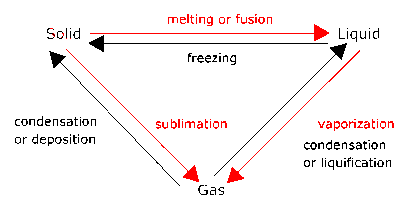
The processes represented by the red arrows require
energy input.
The processes represented by the black arrows release energy.
Melting is a phase transition.
Ice and water are different phases of the same substance. At atmospheric
pressure ice exists at temperatures below 0 oC. If we want to
raise the temperature of 1 kg of ice by 1 degree Celsius, say from -5 oC
to - 4 oC, we need 0.49 kcal of heat. But if we want to melt
1 kg of ice at 0 oC, we need 80 kcal of heat. This heat is
used to break chemical bonds and is converted into potential energy. It is
called the latent heat of melting or latent heat of
fusion (Lf). Latent heat is heat that changes the phase of
a substance without changing its temperature. As we add heat to a mixture of
water and ice, heat will flow from the water into the ice at 0 oC
until all the ice has melted. If we could thermally isolate the mixture, it
would reach thermal equilibrium at 0 oC, and no more ice would melt.
If heat is removed from water at 0 oC it freezes into ice rather than
becoming colder. Freezing releases the
latent heat of fusion.
Freezing releases large amounts of heat. The
temperature of a large body of water is therefore very stable
near the freezing point. Winter weather is moderated to a
great extent by this factor. Winter temperatures will stay
near 0o C until all the local water is frozen.
But it also takes a lot of energy to melt ice, and therefore
local temperatures do not increase much until the ice has
melted.
Boiling is a phase transition. Water and
steam are different phases of the same substance. At atmospheric pressure
water exists at temperatures between 0 oC and 100 oC. If
we want to raise the temperature of 1 kg of water by 1 degree Celsius, say from
50 oC to 51 oC, we need 1 kcal of heat. But if we
want to boil 1 kg of water at 100 oC and turn it into steam we need
540 kcal of heat. This is called the latent
heat of vaporization (LV). It is used to break the
bonds that hold the water molecules together in the liquid. The boiling
temperature of water depends on the pressure. The lower the pressure, the lower
is the boiling temperature. At lower pressure the molecules need less kinetic
energy to escape from the liquid.
Boiling water does not raise the temperature
beyond 100 oC until all the water has vaporized. The steam we see is
not the water vapor, which is invisible, but a collection of small water
droplets, which form when gaseous water vapor cools.
The phases of matter
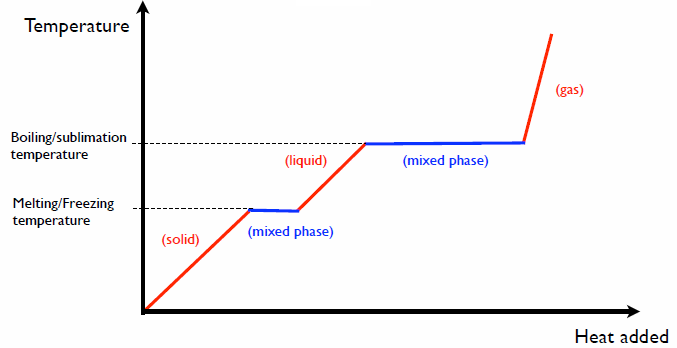
Adding heat in in a given phase changes the temperature.
Specific heat c: ΔQ = c*m*ΔT
Adding heat during a phase change converts from one phase to another phase
without changing the temperature.
Latent heat L: ΔQ = m*L
How much thermal energy is required to change a 40 g ice cube from a solid at -10 oC to steam at 110 oC?
Solution:
If 90 g of molten lead at its melting point
of 327.3 oC is poured into a 300 g casting
form made of iron and initially at 20 oC, what is the final
temperature of the system? Assume no energy is lost
to the environment.
Lead: latent heat Lf
= 2.45*104
J/kg, specific heat c = 128 J/(kgoC).
Iron: specific heat c = 448
J/(kgoC).
Solution:
 Orange
growers in Florida spray their trees with water when they expect a freeze. Why
does this work?
Orange
growers in Florida spray their trees with water when they expect a freeze. Why
does this work?
Answer:
The temperature of a substance is a measure of the average kinetic energy of the atoms or molecules that make up the substance. But not all particles have the same kinetic energy, they have a distribution of energies. Some particles in a liquid or solid may have enough kinetic energy to break the chemical bonds and leave the substance. The liquid is evaporating and the solid is subliming. When the particles with the highest kinetic energy leave the substance, the average kinetic energy of the remaining particles decreases. The temperature of the substance therefore decreases. Evaporation cools the substance.
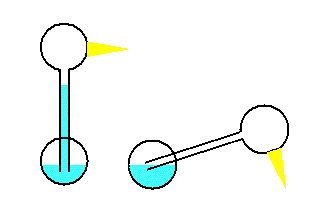
 A toy that demonstrates the cooling process of
evaporation is the dippy bird. The dippy bird is made by blowing a
glass tube that flows into a bulb like the neck of a funnel. This
upper bulb becomes the head of the bird. A second bulb becomes the
body. The tube extends almost to the bottom of this lower
bulb, like a straw into a soft drink.
A toy that demonstrates the cooling process of
evaporation is the dippy bird. The dippy bird is made by blowing a
glass tube that flows into a bulb like the neck of a funnel. This
upper bulb becomes the head of the bird. A second bulb becomes the
body. The tube extends almost to the bottom of this lower
bulb, like a straw into a soft drink.
The bird is filled with a liquid which has a high vapor pressure.
The bird's head is covered with fuzz, which gives a large area for
evaporation. When the head is wet, evaporation causes cooling
and condensation of the gas inside the head bulb, and the pressure
drops. This causes the fluid to creep up the neck to the area
of lower pressure. The center of gravity shifts to the head
end of the bird. The bird tips into the beaker of water where
the fuzz becomes wet again. But as the bird reaches its
maximum lean, the bottom end of the tube sticks out of the fluid,
and the fluid can flow out of the tube back into the bottom bulb.
This shifts the center of gravity back and mixes the gas. The process continues
as long as there is water in the beaker.
 External
link: The
engineering of the drinking bird (Youtube)
External
link: The
engineering of the drinking bird (Youtube)
Terminology for different phase changes:
| Liquid to gas: | Evaporation |
|---|---|
| Gas to liquid: | Condensation |
| Solid to gas: | Sublimation |
| Gas to solid: | Deposition |
Molecules can leave liquid water through evaporation, but molecules also can re-enter the liquid water if there is water vapor in the surrounding air. We call this phenomenon condensation. The relative humidity is the ratio of the rate of condensation to the rate of evaporation. If twice as many molecules leave the liquid than are returning to the liquid, then the relative humidity is 0.5 or 50%. The relative humidity depends on the temperature and on the density of the water vapor in the air. The higher the temperature, the higher is the average kinetic energy of the molecules and therefore the rate at which they are leaving. The higher the density of water vapor in the air, the higher is the rate the molecules are returning.
In an enclosed container at a given temperature, an equilibrium will be
reached, and the number of water molecules that leave the water's surface will
equal the number that return per unit time. The pressure of the water vapor at
equilibrium is called the saturated vapor pressure. At a
higher temperature more molecules can escape the surface and the saturated vapor
pressure is higher. The temperature at which the vapor pressure is equal
to the atmospheric pressure is called the boiling point. If the liquid is
open to the air, then the vapor pressure is seen as a partial pressure along
with the other constituents of the air.
At temperatures below 100 oC, water, left open to the
air, evaporates slowly from its surface. Its vapor pressure is much lower than
atmospheric pressure. Microscopic bubbles also form in the interior,
but these tiny bubbles of water vapor, with a low pressure equal to the vapor
pressure, are immediately suppressed by the much higher pressure of the
atmosphere pressing down on the liquid's surface. When the water is heated and
its temperature reaches 100 oC, its vapor pressure reaches the
pressure of the surrounding air. Now bubbles that form by evaporation in the
interior of the liquid are no longer suppressed. They grow to large sizes, rise
to the surface, and release their vapor to the air. That sometimes explosive
evaporation that starts in the interior of the liquid is called boiling. The bubbles
are water vapor, possibly mixed with a small amount air that used to be
dissolved the water. Previously dissolved air also produces the tiny bubbles
that appear at the start of the heating process.
Knoxville weather is often hot and humid in the summer. Under those
conditions, why is the time after sunrise the most comfortable time of the day?
Why is it not the time just after sunset, when the temperature is starting to
drop slightly?
Answer:
Just after sunrise, when the temperature is rising, the rate of evaporation
increases. The amount of water vapor in the air, however, is still low.
The rate of condensation and the relative humidity therefore are quite low.
Sweat on the skin therefore will evaporate. Evaporation cools the remaining
sweat and, through conduction, the skin. As the density of water vapor in
the air increases, the net rate of evaporation decreases, because more and
more molecules are entering the liquid. The sweat does no longer cool the
skin efficiently. Just after sunset, when the temperature drops, the
humidity increases even more, because the rate of evaporation drops, but the
density of the water vapor in the air is still high.
 Would the "Dippy Bird" still work, if the humidity were 100%?
Would the "Dippy Bird" still work, if the humidity were 100%?
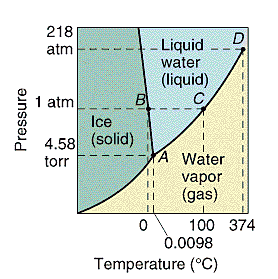
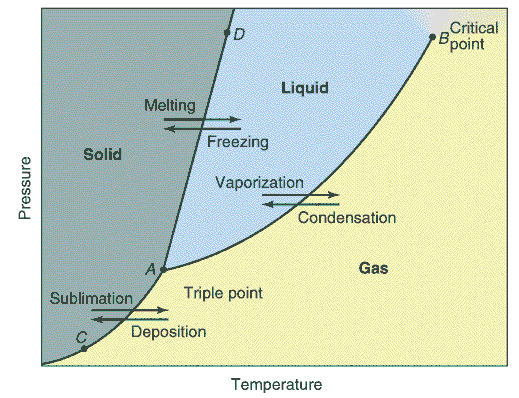 Every substance can exist as a solid, liquid, or gas.
A phase diagram is a plot of all the equilibrium curves between
any two phases on a pressure temperature diagram. The exact shape of
the phase diagram depends on the substance. There is only one point on
the diagram where all three phases of a pure substance are at
equilibrium. This is called the triple point.
Every substance can exist as a solid, liquid, or gas.
A phase diagram is a plot of all the equilibrium curves between
any two phases on a pressure temperature diagram. The exact shape of
the phase diagram depends on the substance. There is only one point on
the diagram where all three phases of a pure substance are at
equilibrium. This is called the triple point.
What is the relationship between heat and temperature? Explain!
Discuss this with your fellow students in the discussion forum!