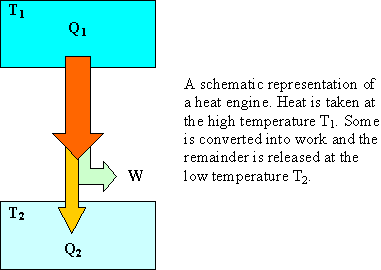
 To convert heat into work, you need at least two places
with different temperatures. If you take in Qhigh at
temperature Thigh you must dump at least Qlow at
temperature Tlow. The amount of work you get out of a
heat engine is W = Qhigh - Qlow. The maximum amount of work you can get out of a
heat engine is the amount you get
out of a reversible engine.
To convert heat into work, you need at least two places
with different temperatures. If you take in Qhigh at
temperature Thigh you must dump at least Qlow at
temperature Tlow. The amount of work you get out of a
heat engine is W = Qhigh - Qlow. The maximum amount of work you can get out of a
heat engine is the amount you get
out of a reversible engine.
Wmax = (Qhigh - Qlow)reversible
= Qhigh - QhighTlow/Thigh
= Qhigh(1 - Tlow/Thigh).
W is positive if Thigh is greater than Tlow.
The efficiency of a heat engine
is the ratio of the work obtained to the heat energy put in at the high
temperature, e = W/Qhigh. The maximum possible
efficiency emax of such an engine is
emax = Wmax/Qhigh = (1 - Tlow
/Thigh) = (Thigh - Tlow)/Thigh.
 Steam engines
Steam engines
A steam engine is a type of heat engine. It takes heat from the
hot steam, converts some of this heat into useful work and dumps the
rest into the colder surrounding air. The maximum fraction of heat
that can be converted into work can be found using the laws of
thermodynamics, and it increases with the temperature difference between
the hot steam and the surrounding air. The hotter the steam and
the colder the air, the more efficient is the steam engine at converting
heat into work.
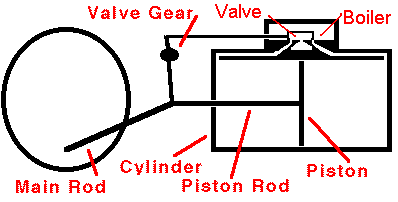
In a typical steam engine a piston moves back and forth inside a
cylinder. Hot, high-pressure steam is produced in a boiler, and
this steam enters the cylinder through a valve. Once inside the
cylinder, the steam pushes outward on every surface, including the
piston. The piston moves. The steam does mechanical work on
the piston and the piston does mechanical work on the machinery attached
to it. The expanding steam transfers some of its thermal energy to
this machinery, so the steam becomes cooler as the machinery operates.
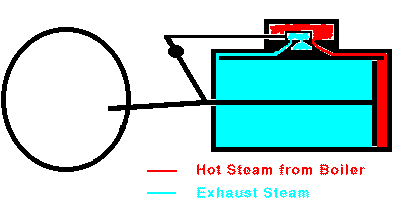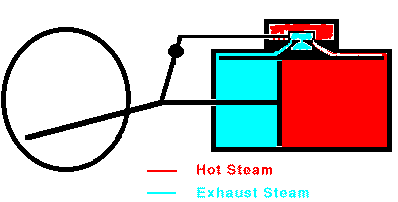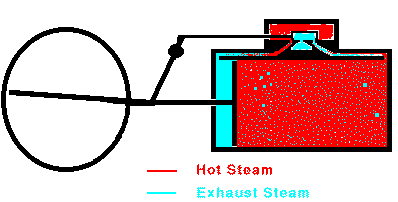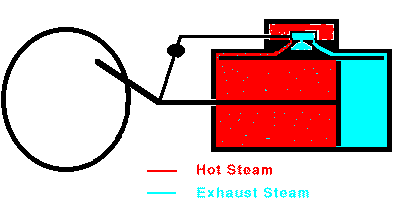 When the piston reaches the end of its range, the valve stops the
flow of steam and opens the cylinder to the outside air. The
piston can then return easily. In many cases, steam is allowed to
enter the other end of the cylinder so that the steam pushes the piston
back to its original position. Once the piston is back at its
starting point, the valve again admits high-pressure steam to the
cylinder and the whole cycle repeats. Overall, heat is flowing
from the hot boiler to the cooler surrounding air and some of that heat
is being converted into mechanical work by the moving piston. The
maximum efficiency of a steam engine is emax = (Tsteam
- Tair)/Tsteam. The actual efficiency
is usually much lower.
When the piston reaches the end of its range, the valve stops the
flow of steam and opens the cylinder to the outside air. The
piston can then return easily. In many cases, steam is allowed to
enter the other end of the cylinder so that the steam pushes the piston
back to its original position. Once the piston is back at its
starting point, the valve again admits high-pressure steam to the
cylinder and the whole cycle repeats. Overall, heat is flowing
from the hot boiler to the cooler surrounding air and some of that heat
is being converted into mechanical work by the moving piston. The
maximum efficiency of a steam engine is emax = (Tsteam
- Tair)/Tsteam. The actual efficiency
is usually much lower.
External link: Steam locomotive (Youtube)
Problem:
What is the maximum
possible efficiency of a steam engine taking in heat at 100 oC
and dumping it at room temperature of approximately 20 oC?
Solution:
- Reasoning:
The maximum efficiency of any heat engine is that of a Carnot engine. emax = (Thigh - Tlow)/Thigh.
- Details of the calculation:
100 oC = 373 K and 20
oC = 293 K. The
maximum possible efficiency is
(Thigh - Tlow)/Thigh
= (373 -
293)/373 = 0.21 = 21%.
 Internal combustion engines
Internal combustion engines
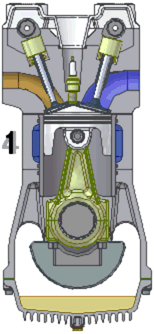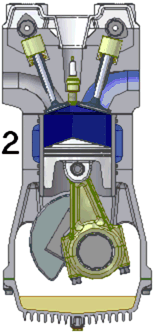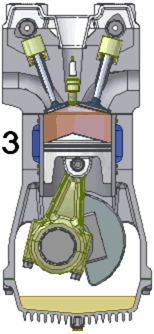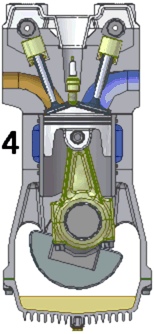
An internal combustion engine burns a mixture of fuel and air.
The most common type is a four-stroke engine. A piston slides in
and out of a cylinder. Two or more valves allow the fuel and the
air to enter the cylinder and the gases that form when the fuel and air
burn to leave the cylinder. As the piston slides back and forth
inside the cylinder, the volume that the gases can occupy changes
drastically.
The process of converting heat into work begins when the piston is
pulled out of the cylinder, expanding the enclosed space and allowing
fuel and air to flow into that space through a valve. This motion
is called the intake stroke or induction stroke. Next, the fuel and
the air mixture are squeezed together by pushing the piston into the
cylinder. This is called the compression
stroke. At the end of the compression stroke, with the
fuel and the air mixture squeezed as tightly as possible, the spark plug
at the sealed end of the cylinder fires and ignites the mixture.
The hot burning fuel has an enormous pressure and it pushes the piston
out of the cylinder. This power stroke is what provides power to the engine and the attached machinery.
Finally, the burned gas is squeezed out of the cylinder through another
valve in the exhaust stroke.
These four strokes repeat over and over again. Most internal
combustion engines have at least four cylinders and pistons. There
is always at least one cylinder going through the power stroke and it
can carry the other cylinders through the non-power strokes. The
maximum efficiency of such an engine is emax = (Tignition
- Tair)/Tignition where Tignition
is the temperature of the fuel-air mixture after ignition. To
maximize the fuel efficiency, you have to create the hottest possible
fuel air mixture after ignition. The highest efficiency that has
been achieved is approximately 50% of emax.
External link: Interal combustion
engine (Youtube)
Problem:
A heat engine absorbs 360 J of thermal energy and performs 25 J of work in
each cycle. Find
(a) the efficiency of the engine and
(b) the thermal energy expelled in each cycle.
Solution:
- Reasoning:
The amount of work you get out of a heat engine is W = Qhigh - Qlow.
The efficiency is e = W/Qhigh.
- Details of the calculation:
Qhigh = 360 J. W = 25 J. Qlow
= Qhigh - W = 335
J.
(a) The efficiency e = W/Qhigh = 6.9%.
(b) The thermal energy expelled is Qlow
= 335 J.
 To convert heat into work, you need at least two places
with different temperatures. If you take in Qhigh at
temperature Thigh you must dump at least Qlow at
temperature Tlow. The amount of work you get out of a
heat engine is W = Qhigh - Qlow. The maximum amount of work you can get out of a
heat engine is the amount you get
out of a reversible engine.
To convert heat into work, you need at least two places
with different temperatures. If you take in Qhigh at
temperature Thigh you must dump at least Qlow at
temperature Tlow. The amount of work you get out of a
heat engine is W = Qhigh - Qlow. The maximum amount of work you can get out of a
heat engine is the amount you get
out of a reversible engine. Steam engines
Steam engines When the piston reaches the end of its range, the valve stops the
flow of steam and opens the cylinder to the outside air. The
piston can then return easily. In many cases, steam is allowed to
enter the other end of the cylinder so that the steam pushes the piston
back to its original position. Once the piston is back at its
starting point, the valve again admits high-pressure steam to the
cylinder and the whole cycle repeats. Overall, heat is flowing
from the hot boiler to the cooler surrounding air and some of that heat
is being converted into mechanical work by the moving piston. The
maximum efficiency of a steam engine is emax = (Tsteam
- Tair)/Tsteam. The actual efficiency
is usually much lower.
When the piston reaches the end of its range, the valve stops the
flow of steam and opens the cylinder to the outside air. The
piston can then return easily. In many cases, steam is allowed to
enter the other end of the cylinder so that the steam pushes the piston
back to its original position. Once the piston is back at its
starting point, the valve again admits high-pressure steam to the
cylinder and the whole cycle repeats. Overall, heat is flowing
from the hot boiler to the cooler surrounding air and some of that heat
is being converted into mechanical work by the moving piston. The
maximum efficiency of a steam engine is emax = (Tsteam
- Tair)/Tsteam. The actual efficiency
is usually much lower. Internal combustion engines
Internal combustion engines