In this studio session you will analyze the performance of a simple heat
engine and review the various ways to state the second law of thermodynamics.
Open a Microsoft Word document to keep a log of your
experimental procedures, results and discussions. Address the points highlighted in blue. Answer all questions.
A heat engine
How can we do work with heat? How can we convert some disordered energy
back into ordered energy?
In this exercise you will analyze data obtained with is a "real" thermal
engine that can be taken through a four-stage expansion and compression cycle
and that can do useful mechanical work by lifting small masses from one height
to another. You will determine the useful mechanical work done by the
engine by measuring the vertical distance y a mass m is lifted. You will
compare this mechanical work Wmech = mgy to the net thermodynamic
work done during a cycle. The pressure as a function of volume is recorded
for one cycle, and the net thermodynamic work done by the engine equals the
enclosed area on the PV diagram.
W = ∑jPjΔVj. We sum the work done during a
large number of small changes of the volume.
 The PASCO TD-8572 Heat Engine/Gas Law Apparatus is used to obtain the data.
The heart of this apparatus is a nearly friction-free piston-cylinder system.
The graphite piston fits snugly into a precision-ground Pyrex cylinder so that
the system produces almost friction-free motion and negligible leakage.
The Heat Engine/Gas Law Apparatus is designed with two pressure ports with
quick-connect fittings for connecting to an air chamber and a pressure sensor
with tubing.
The PASCO TD-8572 Heat Engine/Gas Law Apparatus is used to obtain the data.
The heart of this apparatus is a nearly friction-free piston-cylinder system.
The graphite piston fits snugly into a precision-ground Pyrex cylinder so that
the system produces almost friction-free motion and negligible leakage.
The Heat Engine/Gas Law Apparatus is designed with two pressure ports with
quick-connect fittings for connecting to an air chamber and a pressure sensor
with tubing.
Your instructor will take you through a cycle of the PASCO TD-8572 Heat Engine.
(For a short video of data acquisition
click here.)
After you have made your measurements, the instructor will also demonstrate two
different types of real working heat engines to you.
Experiment
Procedure:
With no mass on the platform, the piston is raised ~4
cm, and the air chamber with tubing is connected to the engine. The piston
stays at a height of ~4 cm.
The computer is set up to record the pressure with respect to some reference
pressure (in units of kPa), the volume
of the gas in the cylinder with respect to some reference volume (in units of cm3), and the position of the
cylinder above its starting position (in units of m) as a function of time and to produce plots of pressure versus volume and position versus time.
Measurement:
- Data acquisition starts.
Click on the thumbnails to see a larger
picture.
(a) The air chamber is placed into ice water.
(b) A 100 g
mass is placed on the platform. The weight of the mass increases the
pressure at constant temperature, and the volume and therefore the height of
the piston decrease by some amount.
(c) The air chamber is placed
into hot water. The temperature rises and the gas expands. The
volume and therefore the height of the piston increase.
(d) When
the volume is no longer increasing, the mass is removed from the platform.
Removing the weight decreases the pressure at constant temperature, and the
volume and therefore the height of the piston increase by some amount.
(e) The air chamber is placed back into the ice water. The
temperature decreases and the gas contracts. The volume and therefore
the height of the piston decrease.
- When the piston has returned to the original position data acquisition
stops. (For a short video of data acquisition
click here.)
- The computer has produced the plots shown below.
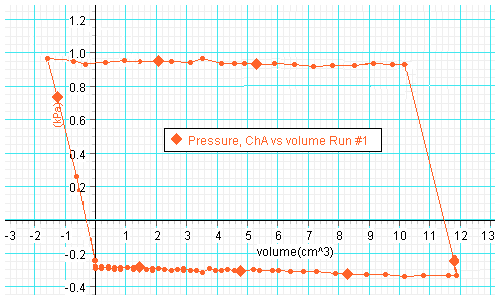
Plot of pressure versus volume:
Plot of piston position versus time:
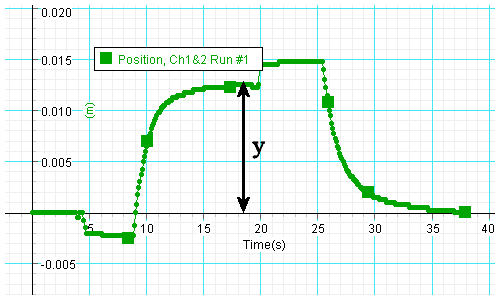
Data analysis:
- Determine the enclosed area on your P-V diagram. (Estimate the area of
the parallelogram by multiplying its width by its height.) The y-axis has
units of kPa and the x-axis has units of cm3.
The area therefore
has units of (kPa)(cm3) = (1000 N/m2)(10-6
m3) = 10-3 Nm = 10-3 J.
Determine
the area in units of J. This area represents the work done by your heat
engine. Record it in a table.
- From the position versus time graph determine the distance y the mass
has been lifted, i.e. the difference in the positions of the platform
just before the mass was put on and just before it was taken off
the platform. (The y-axis in this graph has units of m.)
- Find the change in potential energy mgy of the mass (in units of J),
which is equal to the mechanical work done on the mass. Record it in a table.
- Calculate the %difference between the two values.
mass
|
Work done by heat engine
from P-V diagram (J) |
Change in potential
energy of mass (J) |
% difference
|
| 0.1 kg |
|
|
|
Paste your table into your Word document and answer the following questions.
- The engine starts with an empty platform and the
air chamber in ice water. What happens to the pressure and the volume when
the mass is placed on the platform?
- What happens to the pressure and the volume as the
air chamber is moved from the cold into the hot water?
- What happens to the pressure and the volume as the
mass is removed from the platform?
- What happens to the pressure and the volume as the
air chamber is moved back into the ice water?
- How does the thermodynamic work compare to the
useful mechanical work? What about conservation of energy?
Exercise
We can state the second law of thermodynamics in various ways.
- Heat cannot, of itself, flow from a cold to a hot object.
- Heat cannot be taken in at a certain temperature with no other change
in the system and converted into work.
- The total entropy of a closed system is always increasing.
How do we connect heat flow and entropy?
Discuss and answer all questions (blue font color).
(a) Assume 50 students registered for a course. You need to report the total
number of students who actually attend. You have every student in attendance sign a list.
- Which of the following would constitute a
macrostate description of the class attendance and which would constitute a
microstate description?
- A list of the names of each student present today.
- The number of students in attendance.
(b) Consider the following macrostates of the class.
- All 50 students are present.
- 25 students are present.
- 1 student is present.
- No students are present.
- Which macrostate(s) would have the highest number of microstates?
- Which macrostate(s) would have the lowest number of microstates?
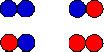 (c) Assume you have two boxes and you have to
place a coin into each box. You toss a coin and place it into box 1
without changing its orientation. The side of the coin facing up can be
either head (blue) or tail (red). Then you toss a second coin and
place it into box 2 without changing its orientation. You are
interested in how many coins end up "head up" or blue. There are 4
different microstates.
(c) Assume you have two boxes and you have to
place a coin into each box. You toss a coin and place it into box 1
without changing its orientation. The side of the coin facing up can be
either head (blue) or tail (red). Then you toss a second coin and
place it into box 2 without changing its orientation. You are
interested in how many coins end up "head up" or blue. There are 4
different microstates.
- How many macrostates are there and what is the multiplicity Ω
of those macrostates, i.e. how many microstates correspond to each macrostate?
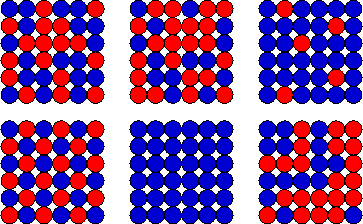 (d) Now assume that you have a 6 by 6 array of
squares. You number the squares from 1 to 36. You toss a coin and place
it onto square 1 without changing its orientation. The side of the coin
facing up can be either head (blue) or tail (red). You keep tossing
coins and placing them in succession onto squares 2 through 36 without
changing their orientation. Each square holds exactly one coin.
(d) Now assume that you have a 6 by 6 array of
squares. You number the squares from 1 to 36. You toss a coin and place
it onto square 1 without changing its orientation. The side of the coin
facing up can be either head (blue) or tail (red). You keep tossing
coins and placing them in succession onto squares 2 through 36 without
changing their orientation. Each square holds exactly one coin.
To specify the microstate of the array after you finished this
process you have to list the color of each square. Many different
patterns (microstates) are possible, such as the ones shown on the
right. Every particular pattern is equally likely to occur. There
are 236 = 6.87*1010 possible patterns and the
probability of observing any particular pattern is 1/236 =
1.46*10-11.
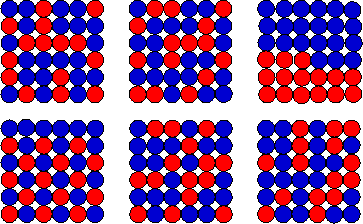 Suppose you are only interested in the macrostate,
i.e. you are only interested in how many squares are red. Most macrostates correspond to many microstates. A few of the allowed
microstate for the "15 red squares" macrostate are shown on the right.
Suppose you are only interested in the macrostate,
i.e. you are only interested in how many squares are red. Most macrostates correspond to many microstates. A few of the allowed
microstate for the "15 red squares" macrostate are shown on the right.
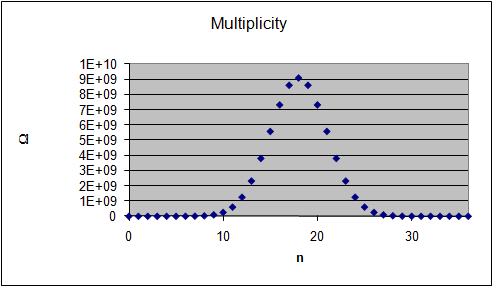
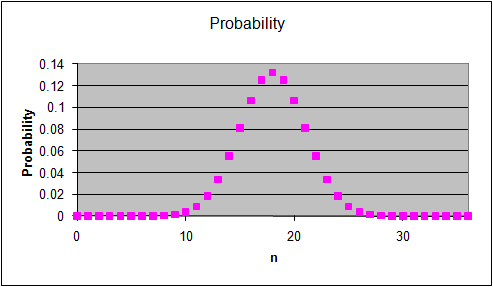
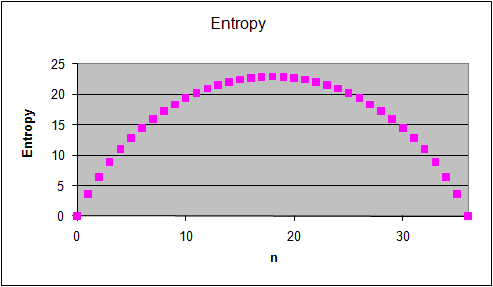
The graphs on the right show the multiplicity Ω for
each "n out of 36" macrostate, the probability of observing this
macrostate and its entropy S = lnΩ.
(The entropy of a macrostate is defined as S = kB
lnΩ, where kB is the Boltzmann constant. Let us measure S in
units of kB and use S = lnΩ.)
Which statement below best describes the
relationship between probability and entropy for the macrostates we are
considering?
- The most probable macrostates have the lowest entropy.
- The most probable macrostates have the highest entropy.
- There is no clear relationship between entropy and probability for these macrostates.
If we examine the probabilities for all of the
macrostates in the system we find that the probability is
- spread evenly among all of the
macrostates.
- spread out among a large
fraction of the macrostates, but with some macrostates having
slightly higher probabilities than others.
- concentrated within a
small fraction of the macrostates centered on a most probable macrostate.
- entirely concentrated into a
single most probable macrostate.
How does this behavior scale with the number of coins N?
- If you have N squares
and N coins, the most probable number "head up" coins is N/2. The
spread of the distribution increases a N½, so the
percentage spread decreases as N-1/2.
- For 36 square, the
spread approximately plus or minus 6, the percentage spreads is
(1/6)*100% or 16.6%. That means that if n differs by more than
16.6% from 18, then it is not very likely to occur.
- If there were N = 1024 squares and coins (just as
there might be 1024 molecules in a gas), then the
percentage spread is (10-12)*100% = 10-10%, that
means in n differs from N/2 by more than 10-10% from 0.5*1024,
it is not very likely to occur.
(e) Consider approximately 1024 gas molecules
in a box. A divider which can conduct heat, for example a rubber diaphragm,
separates the box into two chambers of equal size. Each chamber contains half
of the molecules. Assume that the internal energy of the gas is (approximately) fixed.
- Describe some possible macrostates of the gas in
the two chambers of the box.
- Describe a low entropy macrostate.
- Describe a high-entropy macrostate.
- In which situation described below is the entropy
of the gas greatest?
- When the energy is spread nearly
evenly among all the molecules and all the molecules are clustered in
small regions of each chamber.
- When the energy is concentrated in
a small number of molecules that are spread nearly evenly throughout the
box.
- When the energy is spread nearly
evenly among all the molecules and all the molecules are spread nearly
evenly throughout the box.
- When the energy is concentrated in
a small number of molecules which are clustered in a small region of one
chamber of the box.
Newton's laws work equally well backwards in time as they do forwards.
If a sequence of motions and interactions leads to an increase in entropy, then
the time-reversed sequence will lead to a decrease in entropy. Both
sequences are allowed by Newton's laws.
- Why does heat, of itself, not flow from a cold to
a hot object, if it is allowed by Newton's laws?
- What, do you think, determines, the rate of
change of the entropy of a system?
Entropy can decrease locally (in a subsystem). Heat can be moved from a
cold place to a hot place. (We all are familiar with refrigerators and
heat pumps.) But it takes ordered energy that comes from outside the
subsystem. For the entropy of a subsystem to decrease, the system as a
whole must not yet have reached a state of maximum entropy.
Convert your log into a lab report.
Name:
E-mail address:
Laboratory 10 Report
- In one or two sentences, state the goal of this lab.
- Make sure you completed the entire lab and answered all parts. Make
sure you show your work and inserted and properly labeled relevant tables
and plots.
- Add a reflection at the end of your report in a short essay format.
Save your Word document (your name_lab10.docx), go to Canvas, Assignments, Lab
10, and submit your document.
 The PASCO TD-8572 Heat Engine/Gas Law Apparatus is used to obtain the data.
The heart of this apparatus is a nearly friction-free piston-cylinder system.
The graphite piston fits snugly into a precision-ground Pyrex cylinder so that
the system produces almost friction-free motion and negligible leakage.
The Heat Engine/Gas Law Apparatus is designed with two pressure ports with
quick-connect fittings for connecting to an air chamber and a pressure sensor
with tubing.
The PASCO TD-8572 Heat Engine/Gas Law Apparatus is used to obtain the data.
The heart of this apparatus is a nearly friction-free piston-cylinder system.
The graphite piston fits snugly into a precision-ground Pyrex cylinder so that
the system produces almost friction-free motion and negligible leakage.
The Heat Engine/Gas Law Apparatus is designed with two pressure ports with
quick-connect fittings for connecting to an air chamber and a pressure sensor
with tubing.






 (c) Assume you have two boxes and you have to
place a coin into each box. You toss a coin and place it into box 1
without changing its orientation. The side of the coin facing up can be
either head (blue) or tail (red). Then you toss a second coin and
place it into box 2 without changing its orientation. You are
interested in how many coins end up "head up" or blue. There are 4
different microstates.
(c) Assume you have two boxes and you have to
place a coin into each box. You toss a coin and place it into box 1
without changing its orientation. The side of the coin facing up can be
either head (blue) or tail (red). Then you toss a second coin and
place it into box 2 without changing its orientation. You are
interested in how many coins end up "head up" or blue. There are 4
different microstates. (d) Now assume that you have a 6 by 6 array of
squares. You number the squares from 1 to 36. You toss a coin and place
it onto square 1 without changing its orientation. The side of the coin
facing up can be either head (blue) or tail (red). You keep tossing
coins and placing them in succession onto squares 2 through 36 without
changing their orientation. Each square holds exactly one coin.
(d) Now assume that you have a 6 by 6 array of
squares. You number the squares from 1 to 36. You toss a coin and place
it onto square 1 without changing its orientation. The side of the coin
facing up can be either head (blue) or tail (red). You keep tossing
coins and placing them in succession onto squares 2 through 36 without
changing their orientation. Each square holds exactly one coin.  Suppose you are only interested in the macrostate,
i.e. you are only interested in how many squares are red. Most macrostates correspond to many microstates. A few of the allowed
microstate for the "15 red squares" macrostate are shown on the right.
Suppose you are only interested in the macrostate,
i.e. you are only interested in how many squares are red. Most macrostates correspond to many microstates. A few of the allowed
microstate for the "15 red squares" macrostate are shown on the right.