


In this studio session you will investigate some thermometric properties of gases. You will also experimentally investigate a change of phase of water.
Open a Microsoft Word document to keep a log of your experimental procedures, results and discussions. Address the points highlighted in blue. Answer all questions.
Substances can exist in solid, liquid or gas phases.
At a constant pressure changes of phase always occur at the same
temperatures for any pure substance.
The temperature is a measure of the
internal, disordered energy of a substance. The
absolute temperature of any substance is proportional to the average
translational kinetic energy associated with the random motion of
the molecules of the substance.
½m<v2> = (3/2)kBT
In SI units the scale of absolute temperature is Kelvin (K). But we use different temperature scales in everyday situations. The Kelvin scale is identical to the Celsius (oC) scale, except it is shifted so that 0 oC equals 273.15 K.
Discussion 1
Consider the experiment described below.
A 1.0 kg mass and a 3.0 kg mass with different initial temperatures are placed together inside a well-insulated container and allowed to come to thermal equilibrium. The insulated container prevents any transfer of energy to or from the environment (including the container itself).
| Mass | 1.0 kg | 3.0 kg |
|---|---|---|
| Initial temperature | 100 oC | 160 oC |
| Specific heat capacity | 440 kJ/(kg oC) | 73.33 kJ/(kg oC) |
| Final temperature | 120 oC | 120 oC |
| ΔT | +20 oC | -40 oC |
Do you agree or disagree with the following statements? Explain why you agree or disagree.
Experiment
The experiment starts by measuring the mass of a Styrofoam cup (and
temperature sensor). Then a quantity of hot water is added, and the
temperature of the water and the mass of the cup and water is measured.
Then ice at its melting temperature
(0 oC) is added until the temperature of the water drops to about 10 oC
and all the ice has melted. The final
temperature of the water and the mass of the cup and water (initial and melted)
is measured.
Note: The scale shows the masses in gram. It shows numbers with one
digit behind the decimal point.
 |
 |
 |
| The mass of the cup is measured at room temperature. |
Hot water has been added and the initial temperature Ti is measure. | Ice has been added and the final temperature Tf is measured. |
Your task:
Determine the latent heat of fusion of ice/water using the measured masses and temperatures and value of the specific heat capacity of water.
Procedure:
| mass of cup (kg) | mcup | |
|---|---|---|
| mass of cup + water (kg) | mcup + mw | |
| mass of cup + water + ice (kg) | mcup + mw + mice | |
| mass of water (kg) | mw | |
| mass of ice (kg) | mice | |
| initial temperature of water (oC) | Ti | |
| final temperature of water (oC) | Tf | |
| latent heat of fusion (kcal/kg) | Lf (measured) | |
| latent heat of fusion (kcal/kg) | Lf (accepted) |
Data Analysis:
Discuss:
The ideal gas law states that for a fixed volume of an ideal gas (PV/T) = nR = constant. Here P and V are the pressure and volume of the gas at absolute temperature T. Theoretical derivations of the ideal gas law neglect the forces that the gas molecules exert on each other. Real gases therefore do not strictly obey the ideal gas law. However, at sufficiently low densities, intermolecular forces do not play a significant role and the ideal gas law becomes increasingly accurate. For instance, at 20 atm pressure and room temperature, the volume of 1 mole of oxygen gas is about 2.3% smaller than predicted by the ideal gas law, but at 1 atm pressure the volume is only about 0.13% smaller.
Remember:
If an equation in physics contains the temperature T, this temperature is always the absolute temperature. In SI units it is always measured in K. If an equation contains a temperature difference ΔT, this temperature difference can be measured in K or oC in SI units, since both scales give the same ΔT.
Exercise
Use an on-line simulation from the University of Colorado PhET group to
verify Boyle's law and Charles' law.
Link to the simulation:
Gas Properties
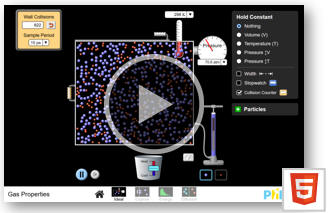
(a) Click on the "Ideal" link. Explore the interface!
(b) Reset the simulation, choose constant volume and add gas to the chamber at 300 K until the pressure is between
20
and 30 atm.
Switch to constant temperature and verify Boyle's law. Boyle's law states that
PV = constant for constant T, or P = constant/V.
Vary the width of the box with the handle.
A plot of P versus 1/V should yield a straight line. If it does, you have
verified Boyle's Law.
Construct an appropriate data table and plot with at least
6 data points. Paste them into your Word document. Add comments. Did you
verify Boyle's law?
[The volume of the box is V = depth * height * width. Depth and height are
fixed, so V = constant*width. In the simulation the constant is 35 nm2.
The width can be measured in units of nanometer (nm).]
(c) Switch to constant pressure and verify Charles' law. Charles' law states V = constant * T for constant P, where T is the absolute temperature. A plot of V versus T should yield a straight line. If it does, you have verified Charles' Law.
Construct an appropriate data table and plot with at least 6 data points. Paste them into your Word document. Add comments. Did you verify Charles' law?
(d) Click the energy link at the bottom of the simulation.
Explore the additional interface options!
Add two species gas to the chamber at 300 K until the pressure
is between 40 and 50 atm.
Compare the average kinetic energies of the two types of particles. Explain your results.
Compare the average speeds of the two types of particles. Explain your results.
Discussion 2
The human body has the ability to regulate its temperature so that it remains very close to 37 oC. If the body is overheating as a result of strenuous exercise, the surface blood vessels dilate to increase the blood flow to the surface areas. Heat is carried by the blood to the surface where it causes the skin temperature to increase. The body also begins to produce sweat. The rate of sweating increases strongly with body temperature above 37oC.
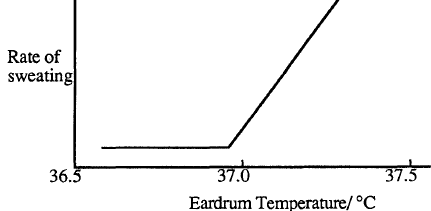
Conduction, evaporation, convection, and radiation can now transfer heat from the skin to the environment. The chart below shows the relative importance of these heat transfer mechanisms at different environmental temperatures.
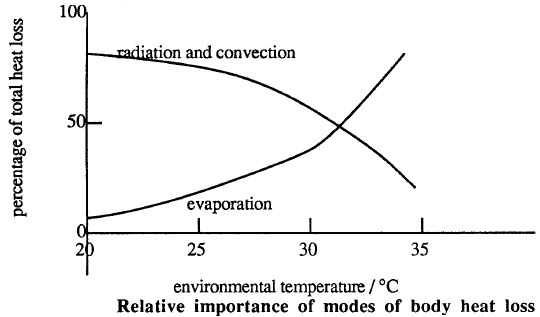
Questions:
The body secrets water on to the skin from sweat glands. As this water evaporates, the latent heat of vaporization is removed from the body. The rate of evaporation increases as the degree of saturation of the surrounding air decreases. In warm humid weather, the rate may be so low that a layer of water accumulates on the skin. In still air, the air near the skin will become saturated and evaporation will stop. Evaporation will increase if this saturated air is continually removed by wind or an artificially produced air stream.
Questions:
Convert your log into a lab report.
Name:
E-mail address:
Laboratory 9 Report
Save your Word document (your name_lab9.docx), go to Canvas, Assignments, Lab 9, and submit your document.