The first law of thermodynamics
Thermal energy is disordered energy. Work is the conversion of one form of energy
into another form. Heat is the exchange of energy between object
because they are in thermal contact with each other. Can we do work with
heat? Can we convert thermal energy into another form of energy? Thermodynamics is the study of the way that one does work with heat.
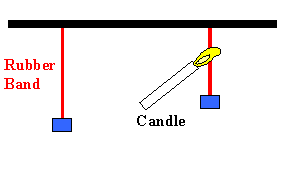
A very simple device that can convert heat into
potential energy is a rubber band. Unlike most other substances, rubber
contracts when heated. We can therefore lift an object by heating a
rubber band. Heat is converted into gravitational potential energy.
Video: A rubber band heat engine
Video:
Longer video clip with explanation
 A simple rubber band can be the heart of an engine converting heat
into electrical energy. We can use heat to make the rubber band
contract. It then can lift water, converting heat into gravitational
potential energy. This gravitational potential energy of the water can
be converted into electrical energy with the help of a turbine
generator. After the load had been lifted, we let the rubber band cool
down. It then has the same internal energy it had before we started the
process. We can repeat this process over and over again. This
certainly is a very inefficient engine. It wastes a large amount of
heat. Can we make the process of converting heat into other forms of
energy more efficient?
A simple rubber band can be the heart of an engine converting heat
into electrical energy. We can use heat to make the rubber band
contract. It then can lift water, converting heat into gravitational
potential energy. This gravitational potential energy of the water can
be converted into electrical energy with the help of a turbine
generator. After the load had been lifted, we let the rubber band cool
down. It then has the same internal energy it had before we started the
process. We can repeat this process over and over again. This
certainly is a very inefficient engine. It wastes a large amount of
heat. Can we make the process of converting heat into other forms of
energy more efficient?
How much work can we possible get out of heat?
Energy conservation limits the amount of work we can get
out of a certain amount of heat. The first law of
thermodynamics state that energy is conserved.
We may express it in the following way:
Change in internal energy of a system
= heat put into the system - work done by the system on its surroundings,
or
ΔU = ΔQ - ΔW.
A system can be anything. It is most convenient if it has well defined boundaries.
- ΔQ is positive if heat flows into the system, negative if
it flows out of the system.
- ΔW is positive if the system does work on its surroundings
and it is negative if work is done on the system.
- The internal energy U is the sum of the kinetic and potential
energy of the atoms and molecules that make up the system. It
is a physical property of the system.
A physical property of a system in a given state can be measured. It depend only on
the state of the system, not on the way the system was put into this
state. For an ideal gas, for example, U = (3/2)NKBT. The
internal energy of the gas depends only on the
number N of gas atoms present and on the temperature T of the gas, not on the way
the gas has reached that temperature.
Problem:
How much heat transfer occurs from a system, if its internal energy decreased
by 150 J while it was doing 30.0 J of work?
Solution:
- Reasoning:
Energy conservation <--> the first law of thermodynamics
increase in internal energy of a system
= heat put into the system -
work done by the system on its surroundings.
- Details of the calculation:
ΔQ = ΔU + ΔW = -150 J + 30 J = -120 J.
120 J is transferred from the system to its environment.
Problem:
A thermodynamic system undergoes a process in which its internal energy
decreases by 500 J. If at the same time 220 J of work is done on the
system, find the thermal energy transferred to or from it.
Solution:
- Reasoning:
Energy conservation <--> the first law of thermodynamics
increase in internal energy of a system
= heat put into the system -
work done by the system on its surroundings.
- Details of the calculation:
ΔU = ΔQ - ΔW.
-500J = ΔQ + 220 J.
ΔQ = -720 J.
Problem:
Suppose a woman does 500 J of work and 9500 J of heat transfer occurs into
the environment in the process. What is the decrease in her internal
energy, assuming no consumption of food
Solution:
- Reasoning:
Energy conservation <--> the first law of thermodynamics
increase in internal energy of a system
= heat put into the system -
work done by the system on its surroundings.
- Details of the calculation:
ΔU = ΔQ - ΔW = -9500 J - 500 J = -10000 J is the decrease
in her internal energy.
Problem:
Assume you are running an electric space heater. Let the space heater
be the system under consideration. It has warmed up and is now running
at a constant temperature. It consumes 500 Watts of electrical power.
How much electrical energy is converted into heat per hour? (1 Watt = 1
J/s)
Solution:
- Reasoning:
Energy conservation <--> the first law of thermodynamics
increase in internal energy of a system
= heat put into the system -
work done by the system on its surroundings.
ΔU = ΔQ - ΔW.
ΔU = 0 since the temperature of the heating element is constant.
Therefore ΔQ = ΔW.
- Details of the calculation:
ΔW is negative, because the work is done on the system by the
power supply in providing electrical energy to the system. ΔQ must be negative. It is
the heat leaving the system. 500 J of heat are leaving the
system per second.
500 J/s * 3600 s = 1.8 MJ of electrical energy are converted into
heat every hour. All the electrical energy put into the system
is converted into heat.
Any form of ordered energy can be completely converted into thermal energy.
Can we reverse the process? Can we take the heat generated by the
space heater or by some other process and completely convert it back to
electrical energy? Can we design an engine
(a device) that accomplices this? The first law of
thermodynamics does not exclude this possibility. It just says that we
will not get more electrical energy out of a system than the energy we
put into the system in the form of heat, assuming ΔU = 0, i.e. the internal energy of the system is the same before and after doing the
work.
 A simple rubber band can be the heart of an engine converting heat
into electrical energy. We can use heat to make the rubber band
contract. It then can lift water, converting heat into gravitational
potential energy. This gravitational potential energy of the water can
be converted into electrical energy with the help of a turbine
generator. After the load had been lifted, we let the rubber band cool
down. It then has the same internal energy it had before we started the
process. We can repeat this process over and over again. This
certainly is a very inefficient engine. It wastes a large amount of
heat. Can we make the process of converting heat into other forms of
energy more efficient?
A simple rubber band can be the heart of an engine converting heat
into electrical energy. We can use heat to make the rubber band
contract. It then can lift water, converting heat into gravitational
potential energy. This gravitational potential energy of the water can
be converted into electrical energy with the help of a turbine
generator. After the load had been lifted, we let the rubber band cool
down. It then has the same internal energy it had before we started the
process. We can repeat this process over and over again. This
certainly is a very inefficient engine. It wastes a large amount of
heat. Can we make the process of converting heat into other forms of
energy more efficient?