Under normal conditions, water exists in one of three phases, the solid phase
(ice), the liquid phase (water), and the gaseous phase (steam). Liquid Water is
an amazing substance. At one atmosphere of pressure, it exists at temperatures
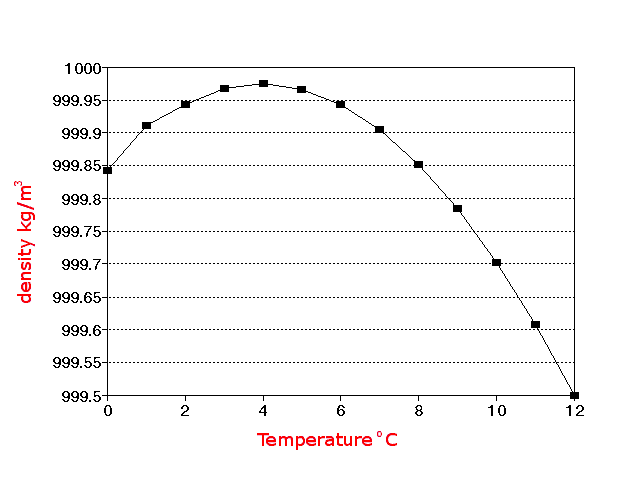
between 0 oC and 100 oC. Over most of this temperature
range (4 oC and 100 oC) its density increases slightly as
the water cools, but for temperatures between 0 oC and 4 oC
its density decreases as it cools.
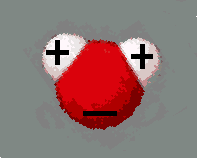 Every water molecule (H2O) consists of
one oxygen atom and two hydrogen atoms. A water molecule has no net
charge because the number of positively charged protons equals the
number of negatively charged electrons in each atom. In the water
molecule, each hydrogen atom is bound to the oxygen atom by a covalent bond. The hydrogen atom
and the oxygen atom share an electron, which has a slightly higher
probability to be closer to the oxygen atom than to the hydrogen
atom. This results in a polar molecule,
in which the oxygen "side" of the molecule has a slight negative
charge, while the hydrogen "sides" have a slight positive charge.
The negative and the positive sides attract
each other, forming hydrogen bonds. These hydrogen
bonds are about twenty times weaker than the covalent bonds between
hydrogen and oxygen atoms. Because water is a polar molecule it
dissolves many inorganic and organic materials. Organisms can
build macromolecules to attract or repel water as needed simply by
varying the charge on side chains.
Every water molecule (H2O) consists of
one oxygen atom and two hydrogen atoms. A water molecule has no net
charge because the number of positively charged protons equals the
number of negatively charged electrons in each atom. In the water
molecule, each hydrogen atom is bound to the oxygen atom by a covalent bond. The hydrogen atom
and the oxygen atom share an electron, which has a slightly higher
probability to be closer to the oxygen atom than to the hydrogen
atom. This results in a polar molecule,
in which the oxygen "side" of the molecule has a slight negative
charge, while the hydrogen "sides" have a slight positive charge.
The negative and the positive sides attract
each other, forming hydrogen bonds. These hydrogen
bonds are about twenty times weaker than the covalent bonds between
hydrogen and oxygen atoms. Because water is a polar molecule it
dissolves many inorganic and organic materials. Organisms can
build macromolecules to attract or repel water as needed simply by
varying the charge on side chains.
Strong inter-molecular forces are the reason that
water molecules are hard to pull apart. The melting point of ice is
higher than for other substances of similar molecular weight, and
the boiling point of water is higher than expected for similar
compounds. Each transition, melting or boiling, requires an input
of thermal energy to break the bonds.
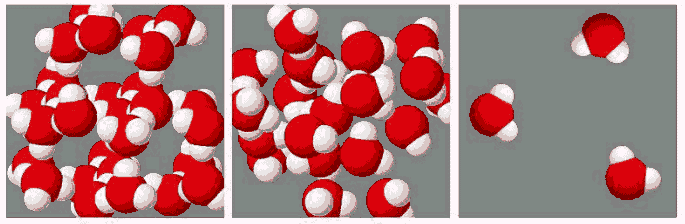
 Water freezes when the molecules have slowed down
enough to develop bonds upon collision. The rate at which freezing
occurs is governed by nucleation and growth. Nucleation is the
formation of small solids in a liquid. Once these "nuclei" have
formed, they become the landing sites for other molecules to attach
onto. The growth rate is the rate at which the radius of a nucleus
grows after formation. It is determined by how many molecules bind
and how many bonds are broken per unit time. Adding a foreign
substance such as salt, sugar, or ethylene glycol to water lowers
the freezing temperature. The foreign substance replaces water
molecules in the liquid and there are fewer water molecules to bind
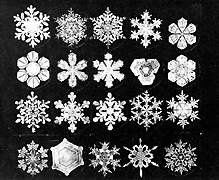
to the nuclei. Fast nucleation rates and/or slow growth rates
result in the formation of many small crystals. Slow nucleation
rates result in the formation of large crystals.
Water freezes when the molecules have slowed down
enough to develop bonds upon collision. The rate at which freezing
occurs is governed by nucleation and growth. Nucleation is the
formation of small solids in a liquid. Once these "nuclei" have
formed, they become the landing sites for other molecules to attach
onto. The growth rate is the rate at which the radius of a nucleus
grows after formation. It is determined by how many molecules bind
and how many bonds are broken per unit time. Adding a foreign
substance such as salt, sugar, or ethylene glycol to water lowers
the freezing temperature. The foreign substance replaces water
molecules in the liquid and there are fewer water molecules to bind
to the nuclei. Fast nucleation rates and/or slow growth rates
result in the formation of many small crystals. Slow nucleation
rates result in the formation of large crystals.
The average distance between the water molecules in ice at
0o C is greater than it is
between water molecules at 4o C. Ice is less dense than water
and therefore floats. Water, like most things in nature, expands when heated
and contracts when cooled except in the temperature range between 4o
Cs and 0o
C. The hydrogen bond gives water this unique
property. In ice, a water molecule has four nearest neighbors to which it is bonded via
hydrogen bonds. The geometry leads to a rigid, rather open hexagonal
structure. When the temperature is raised above the melting point,
jostling begins to destroy this open hexagonal structure. Liquid water has
a partially ordered structure in which hydrogen bonds are constantly being
formed and broken. In liquid water each molecule is hydrogen bonded on the
average to 3.4 other water molecules. Molecules are now allowed into the open spaces of the hexagonal
structure. This allows the molecules to move closer to each other and
increases the density. As the temperature increases towards
4o C, molecules move through the liquid so fast
that fewer bonds are formed at any one time, shortening the average time each
bond exists and increasing the density even further. At
atmospheric pressure the temperature of greatest density is
4o C.
Water (H2O)
|
| Specific heat of ice |
0.49 kcal/(kgoC) |
| Latent heat of fusion |
80 kcal/kg |
| Specific heat of water |
1 kcal/(kgoC) |
| Latent heat of vaporization |
540 kcal/kg |
| Specific heat of steam |
0.48 kcal/(kgoC) |
External link: States of matter simulation from the University of Colorado PhET group
 Every water molecule (H2O) consists of
one oxygen atom and two hydrogen atoms. A water molecule has no net
charge because the number of positively charged protons equals the
number of negatively charged electrons in each atom. In the water
molecule, each hydrogen atom is bound to the oxygen atom by a covalent bond. The hydrogen atom
and the oxygen atom share an electron, which has a slightly higher
probability to be closer to the oxygen atom than to the hydrogen
atom. This results in a polar molecule,
in which the oxygen "side" of the molecule has a slight negative
charge, while the hydrogen "sides" have a slight positive charge.
The negative and the positive sides attract
each other, forming hydrogen bonds. These hydrogen
bonds are about twenty times weaker than the covalent bonds between
hydrogen and oxygen atoms. Because water is a polar molecule it
dissolves many inorganic and organic materials. Organisms can
build macromolecules to attract or repel water as needed simply by
varying the charge on side chains.
Every water molecule (H2O) consists of
one oxygen atom and two hydrogen atoms. A water molecule has no net
charge because the number of positively charged protons equals the
number of negatively charged electrons in each atom. In the water
molecule, each hydrogen atom is bound to the oxygen atom by a covalent bond. The hydrogen atom
and the oxygen atom share an electron, which has a slightly higher
probability to be closer to the oxygen atom than to the hydrogen
atom. This results in a polar molecule,
in which the oxygen "side" of the molecule has a slight negative
charge, while the hydrogen "sides" have a slight positive charge.
The negative and the positive sides attract
each other, forming hydrogen bonds. These hydrogen
bonds are about twenty times weaker than the covalent bonds between
hydrogen and oxygen atoms. Because water is a polar molecule it
dissolves many inorganic and organic materials. Organisms can
build macromolecules to attract or repel water as needed simply by
varying the charge on side chains.

 Water freezes when the molecules have slowed down
enough to develop bonds upon collision. The rate at which freezing
occurs is governed by nucleation and growth. Nucleation is the
formation of small solids in a liquid. Once these "nuclei" have
formed, they become the landing sites for other molecules to attach
onto. The growth rate is the rate at which the radius of a nucleus
grows after formation. It is determined by how many molecules bind
and how many bonds are broken per unit time. Adding a foreign
substance such as salt, sugar, or ethylene glycol to water lowers
the freezing temperature. The foreign substance replaces water
molecules in the liquid and there are fewer water molecules to bind
to the nuclei. Fast nucleation rates and/or slow growth rates
result in the formation of many small crystals. Slow nucleation
rates result in the formation of large crystals.
Water freezes when the molecules have slowed down
enough to develop bonds upon collision. The rate at which freezing
occurs is governed by nucleation and growth. Nucleation is the
formation of small solids in a liquid. Once these "nuclei" have
formed, they become the landing sites for other molecules to attach
onto. The growth rate is the rate at which the radius of a nucleus
grows after formation. It is determined by how many molecules bind
and how many bonds are broken per unit time. Adding a foreign
substance such as salt, sugar, or ethylene glycol to water lowers
the freezing temperature. The foreign substance replaces water
molecules in the liquid and there are fewer water molecules to bind
to the nuclei. Fast nucleation rates and/or slow growth rates
result in the formation of many small crystals. Slow nucleation
rates result in the formation of large crystals.