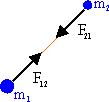
 The force F21, which the particle with mass
m2 exerts on the particle with mass m1, is equal to -F12,
according to Newton's third law. The gravitational force is always
attractive.
The force F21, which the particle with mass
m2 exerts on the particle with mass m1, is equal to -F12,
according to Newton's third law. The gravitational force is always
attractive.In this class we continue to study matter and its interaction. The interactions we have considered so far are gravity and contact forces, such as elastic forces, normal forces, and friction and drag. On a microscopic scale, these contact forces are electromagnetic forces. The electromagnetic force is responsible for the atomic and molecular interactions that determine the properties of inanimate matter and living organisms. But on the atomic scale, how matter behaves as the result of the electromagnetic interactions is predicted by quantum mechanics, not classical mechanics.
During the first 3/4 of the class we will study the classical model of electromagnetic interactions. It works well describing many everyday phenomena. But scientific models can change as our knowledge changes. They are made by people for people, to help us understand observable features of of the world around us. Starting near the beginning of the 20th century new instruments began revealing the behavior of matter on an atomic and sub-atomic scale, and the classical model did not agree with the observations. During the final 1/4 of the class we will be introduced to quantum mechanics, our current model describing the world on the atomic and subatomic scale.
The idea that matter is made of atoms was suggested by the Greek philosopher
Democritus, who lived in the fifth century BC. Experiments by chemists
in the 19th century gave strong evidence that this indeed is the case. In
the 20th century the modern picture of the atom emerged.
Approximately ninety stable atoms make up all matter on earth. An atom
consists of a nucleus surrounded by electrons. The nucleus is a composite
particle, made up of nucleons (protons and neutrons). The electrons are
thought to be elementary particles.
Protons, neutrons, and electrons have an intrinsic property called mass.
| proton mass: | mp = 1.673*10-27 kg |
| neutron mass: | mn = 1.675*10-27 kg |
| electron mass: | me = 9.109*10-31 kg |
Nearly all the mass of an atom is concentrated in the nucleus. The mass of a nucleon is approximately 2000 times larger than the mass of an electron.
Mass is an additive quantity. The mass of a composite object is the sum of the masses of all its constituent. The mass of all particles has the same sign, which is chosen to be positive. When adding masses, the sum is always greater than the mass of the individual constituents, there is no cancellation.
Massive objects have inertia. It takes a force to change their state of
motion. All massive object interact via the
gravitation force. A particle with mass m1
exerts a force F12
on a particle with mass m2. Newton's law of gravitation
gives this force as
F12 = (-G m1m2/r122)
(r12/r12).
Here r12 is the distance between particles 1 and 2, and (r12/r12) is the
unit vector pointing
from particle 1 to particle 2.
G is the gravitational constant, G = 6.67*10-11 Nm2/kg2.
Unit vectors: A unit vector is a vector of magnitude 1. It has no units and is used as a direction indicator. A unit vector pointing in the x-direction has a x-component of 1 and y- and z- components of zero. It is denoted by i or (x/x). Similarly, a unit vector pointing in the y-direction is denoted by j or (y/y), and a unit vector pointing in the z-direction is denoted by k or (z/z).
 The force F21, which the particle with mass
m2 exerts on the particle with mass m1, is equal to -F12,
according to Newton's third law. The gravitational force is always
attractive.
The force F21, which the particle with mass
m2 exerts on the particle with mass m1, is equal to -F12,
according to Newton's third law. The gravitational force is always
attractive.
The magnitude of the gravitational force between two masses decreases as one over the square root of the distance between their centers. We treat an object's weight near the surface of the earth as constant, because its distance from the center of the earth stays roughly constant.
Why do we not notice that inverse square dependence near the surface of Earth?
The radius of Earth is R = 6368 km. If you climb a 1000 m high mountain,
your distance from the center of the earth changes by (1/6368)*100 % = 0.016 %
and the magnitude of the gravitational force acting on you changes by (1/6368)2*100
% = 2.4*10-6 %. For all objects near the surface of Earth the
distance from the center is nearly constant, and the magnitude of the
gravitational force vector is therefore approximately constant and equal to
GMearthmobject/R2
= mobjectg, with g = GMearth/R2
= 9.8 m/s2. Over small distances, when the curvature of the earth's
surface can be neglected, the direction of the gravitational force vector is
also nearly constant. It points straight downward towards the center of the
earth. The force of gravity acting on an object is called its weight.
Earth and Moon attract each other via the gravitational force. Which force diagram correctly represents the magnitude and direction of the force on each of these objects?
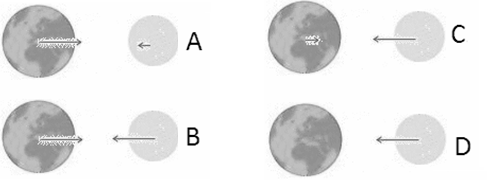
Discuss this with your fellow students in the discussion forum!
Review Newton's laws of motion.
Newton's third law:
For every force that an object exerts on a second object, there is a force equal in magnitude but opposite in direction exerted by the second object on the first object. Forces are the result of interactions.
If you see a picture of the forces two interacting objects exert on each other, the arrows ALWAYS have to have the same length, and point on opposite directions, no matter the details of the objects and the interaction.
Protons and electrons have an another intrinsic property called charge.
Charge is also an additive quantity. The charge of a composite object is the sum of the charges of all its constituent. However, there are two kinds of charge, which we label positive and negative. When adding charges, we can have cancellation. The SI unit of charge is C = Coulomb. The protons and electron charge are
qproton = 1.602*10-19 C, qelectron = -1.602*10-19 C.
The charge of a fundamental particle may be positive or negative, but its magnitude is always an integer multiple of the fundamental quantity qe = 1.6*10-19 C. Unlike mass, charge is quantized. The electric charge of the proton is positive, the charge of the electron is negative. Each electron has a charge of -qe, each proton has a charge of +qe. Neutrons have no charge. The net charge of a system of particles is the sum of the charges of all the particles in the system. If we combine equal amounts of positive and negative charge we obtain zero net charge. The net charge of a closed system is a conserved quantity. Net charge cannot be created or destroyed. We say that charge is conserved.
Atoms are neutral particles. They have no net charge. The charge of the nucleus is exactly canceled by the charge of the electrons. We have exact cancellation, because charge is quantized. The "atomic number" Z of an atom gives the number of protons in the nucleus. The number of electrons in a neutral atom equals the number of protons. The charge of the nucleus is Zqe. The "atomic mass" A gives the total number of nucleons. The number of neutrons is A - Z. Chemical and structural properties of matter are determined by the way electrons are arranged in various atoms and how atoms combine to make molecules or other structures. The number of atoms in ordinary matter is extremely large. For example, 18 grams of water consist of about 6*1023 atoms of oxygen and twice that many atoms of hydrogen. Since a neutral atom is made up of an equal number of protons and electrons, its net charge is zero. The net charge of ordinary matter, composed of neutral atoms, is zero. We only become aware of electrostatic interactions between macroscopic objects when the positive and negative charges on the objects do not exactly cancel, leaving a net charge on the objects.
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. Ions in gases are usually produced in collisions with photons, free electrons, or other atoms and molecules. They are usually highly reactive. Ions also form in liquids. Given the interaction with the molecules of the liquid, ions form if it is energetically favorable to transfer an electron from one type of atom or molecule to another. These ions are usually quite stable.
Estimate the total amount of positive charge in a 60 kg person.
Solution:
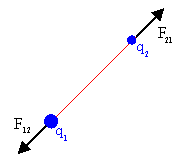 All charged particles interact via the Coulomb force.
A particle with charge q1 exerts a force F12
on a particle with charge q2. Coulomb's law
gives this force as
All charged particles interact via the Coulomb force.
A particle with charge q1 exerts a force F12
on a particle with charge q2. Coulomb's law
gives this force as
F12 = (keq1q2/r122)
(r12/r12).
The constant ke is ke = 9*109 Nm2/C2.
It is often written as ke = 1/(4πε0), where ε0
= 8.85*10-12 C2/(Nm2) is called the
permittivity of free space.
The force F21, which the particle with
charge q2
exerts on the particle with charge q1, is equal to -F12,
according to Newton's third law. Two positively charged particles
repel each other. Two negatively charged particles repel each
other. But a positively charged particle and a negatively charged particle
attract each other.
What is the net force between two objects, each with mass 1 kg and charge +1 C, positioned on the x-axis at x = -0.5 m and x = 0.5 m, respectively?
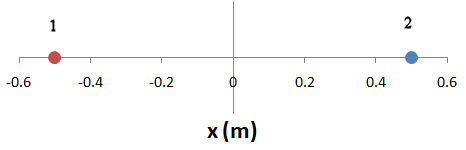
Solution:
Compare the strengths of the electric and the gravitational force between a proton and an electron.
Solution:
When considering the interactions of atomic constituents, gravity is entirely negligible. It becomes only important on a very large scale. This is because the masses of all the constituents of ordinary matter add. Because we live on the surface of a very massive object, we are very aware of the gravitational force. We usually do not observe the electric force between macroscopic objects because ordinary matter is overall electrically neutral. But on a microscopic scale gravity can be neglected and the electric force dominates.
A and B represent objects with -2 and +1 unit of charge respectively. Choose the pair of force vectors that correctly compare the electric force on A (caused by B) with the electric force on B (caused by A).
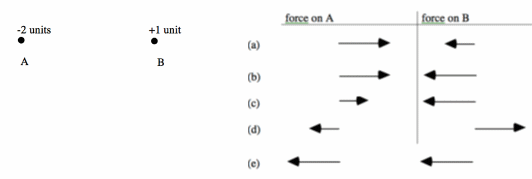
Discuss this with your fellow students in the discussion forum!
Compare the gravitational force and the Coulomb force laws.
The total force on an object is the vector sum of all the forces acting on it. The total electric force is the vector sum of all the electric forces acting on the object. This is called the principle of superposition. To review how to add vectors, explore this link.
External link: Vectors - Fundamentals and Operations
If we have 4 charged particles, with charges q1, q2, q3,
and q4, respectively, then the total electric force acting on
particle 1 is
F1 = F21 +
F31
+ F41
=
-(keq1q2/r122)
(r12/r12) - (keq1q3/r132)
(r13/r13) - (keq1q4/r142)
(r14/r14).
Consider 3 positive charges q at the vertices of an equilateral triangle. Each side has length l. Find the total force on each of the charges.
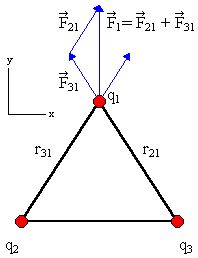
Solution:
Charge cannot be created or destroyed, but charge can be separated. In atoms, the outermost electrons are bound to the nucleus by the weakest net forces. They have the largest distance from the nucleus, and they are also repelled by the inner electrons. Some atoms attract their outermost electrons more strongly than other atoms. For example, atoms in rubber attract their outermost electrons more strongly than atoms in wool. When a rubber rod is rubbed with wool, electrons near the surface are attracted more strongly to the rubber than to the wool, and some electrons are transferred from the wool to the rubber. The rubber becomes negatively charged and the wool becomes positively charged. When enough electrons have been transferred, then the wool becomes more attractive and the rubber more repulsive to additional electrons, and the transfer stops. This phenomenon is called triboelectricity or contact electricity.
| ▲ less attractive to electrons (becomes positively charged) |
|---|
| Rabbit's fur |
| Glass |
| Wool |
| Cat's fur |
| Silk |
| Cotton |
| Wood |
| Amber |
| Rubber |
| Metals (Cu, Ni, Co, Ag) |
| Metals (Pt, Au) |
| Celluloid |
| ▼ more attractive to electrons (becomes negatively charged) |
The triboelectric sequence classifies materials according to the ease with
which they become electron donors or acceptors.
Please explore this
simulation:
Balloons and Static Electricity
You can use the Balloon simulation to show repulsion, but it is a little
tricky. You have to charge one balloon and then charge the
other one. Then the second one repels the first one and vice versa.
Remove the wall.
Every force that we experience in everyday life, except for the gravitational pull of the earth, is electromagnetic in nature. Electromagnetic interactions govern chemistry, determine the strength of materials, and produce light. Electrostatics, describing the interaction between static charged objects, is a part of classical electromagnetic theory. Electrostatics is the basis of several industrial processes. The unintentional separation of positive and negative charges can lead to different types of industrial accidents by producing sparks. Electrostatics forces are also important in many life processes.
If you miss having regular lectures, consider this video lecture.
Lecture 1: What holds our world together?