A LASER (Light Amplification by Stimulated Emission of Radiation) is capable
of producing an intense beam of photons having identical scalar and vector
properties (frequency, phase, direction and polarization). As a result, a
laser beam can be bright, monochromatic, coherent, and unidirectional.
Quantum mechanics predicts that all atomic and molecular systems are
characterized by discreet energy levels. (Confinement leads to quantization.)
The state corresponding to the lowest possible energy level is termed the
ground
state and the states corresponding to the other levels are termed
excited
states.
In a dense medium, such as a solid, liquid, or high pressure gas, frequent
collisions between the atoms or molecules cause transitions between energy
levels. Optically allowed transitions can also change the energy of the
system. An optically allowed transition between energy levels involves the
absorption or emission of a photon with frequency f, such that hf = ∆Eif, where
∆Eif is the energy difference
between the initial and final energy level and h is Planck's constant. If
the angular momentum quantum number li of the initial state and the
angular momentum quantum number lf of the final state differ by 1,
i.e. if ∆l = ±1, then a transition involving
a photon is very likely. If ∆l ≠ ±1, then a
transition involving a photon is much less likely.
If no lower lying state
satisfying ∆l = ±1 exists, an excited state
is called metastable.
∆l = ±1 is called a
selection rule. If this selection rule is not satisfied, an
optical transition is forbidden (unlikely).
The selection rule
∆l = ±1 is a consequence of the photon
being a spin 1 particle. Each photon either delivers or carries away one
unit of intrinsic angular momentum. Angular momentum conservation can be
satisfied if the orbital angular momentum of the atom also changes by one unit.
Angular momentum is a vector. Adding two vectors can yield a
resultant vector with a larger or smaller magnitude, depending on the relative
orientation of the two vectors.
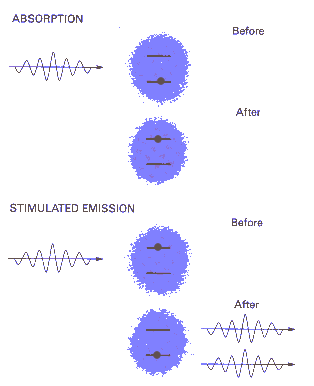 An atom or molecule can emit a photon via
spontaneous emission or
stimulated emission. Atoms and
molecules in excited states randomly emit single photons in all
directions according to statistical rules via spontaneous emission. In
the process of stimulated emission, a photon of energy hf perturbs an
excited atom or molecule and causes it to relax to a lower level,
emitting a photon of the same frequency, phase, and polarization as the
perturbing photon. Stimulated emission is the basis for photon
amplification and it is the fundamental mechanism underlying all laser
action.
An atom or molecule can emit a photon via
spontaneous emission or
stimulated emission. Atoms and
molecules in excited states randomly emit single photons in all
directions according to statistical rules via spontaneous emission. In
the process of stimulated emission, a photon of energy hf perturbs an
excited atom or molecule and causes it to relax to a lower level,
emitting a photon of the same frequency, phase, and polarization as the
perturbing photon. Stimulated emission is the basis for photon
amplification and it is the fundamental mechanism underlying all laser
action.
Consider the simple case of a two-level system with
lower level 1 and upper level 2. The rate of absorption and stimulated
emission depends on the number of photons present. Quantum mechanics
lets us calculate those rates. The probability that the system in level
1 absorbs a photon is equal to the probability that the system in level
2 emits a photon via stimulated emission. The rate of spontaneous
emission, however, is independent of the number of photons present.
Stimulated emission becomes much more likely than
spontaneous emission if many photons are present.
The competition between absorption, stimulated emission, and
spontaneous emission defines the criteria for laser action.
 Consider the simple case of a two-level system with
lower level 1 and upper level 2. Let N1 be the number
density of atoms in level 1 and N2 be the number density in
level 2.
Consider the simple case of a two-level system with
lower level 1 and upper level 2. Let N1 be the number
density of atoms in level 1 and N2 be the number density in
level 2.
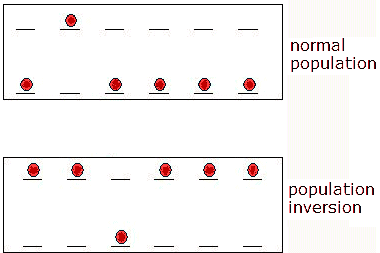
In thermal equilibrium N1 > N2.
Therefore a resonant photon is more likely to be absorbed than to stimulate
emission. But if N2 > N1 there is the
possibility of average overall amplification for an array of photons
passing through a volume of atoms of the two-level system. This
situation is termed population inversion,
since under normal conditions N1 > N2.
Spontaneous emission depletes N2, producing
unwanted photons with random phases, propagation directions, and
polarizations. Because of loss associated with spontaneous emission and
other losses associated with the laser cavity, each laser is
characterized by a minimum value of N2 - N1,
termed the threshold inversion. Only
if N2 - N1 is greater than the threshold inversion
do we see laser action.
 There are several ways of
pumping a system to produce a
population inversion. It is, however, impossible to optically pump a
two-level system, since once N2 = N1 the induced
transition rate equals the absorption rate. Three or four levels are
needed to pump the system and produce the population inversion required
for laser action. Let us investigate the pumping of a four-level system.
There are several ways of
pumping a system to produce a
population inversion. It is, however, impossible to optically pump a
two-level system, since once N2 = N1 the induced
transition rate equals the absorption rate. Three or four levels are
needed to pump the system and produce the population inversion required
for laser action. Let us investigate the pumping of a four-level system.
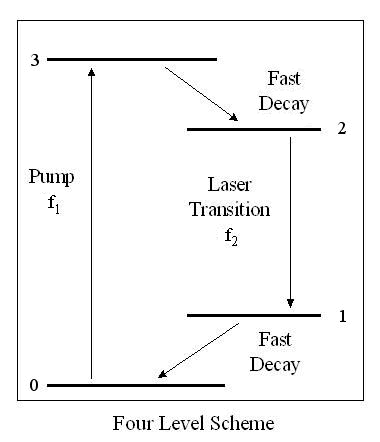
An atom is first excited by optical, electrical, or
other means through the "pump transition" from a starting level "0" to a
temporary level or group of levels "3". The atom quickly relaxes
to a level "2", which may or may not be metastable. Stimulated emission occurs from level
"2" to level "1". Level "1" quickly decays back to level "0", so
that absorption from level "1" to level "2" is unlikely. At any
given time more of the atoms are at level "2" than at level "1", there
is a "population inversion". The rate of stimulated emission will
exceed the absorption rate resulting in an optical amplifier.
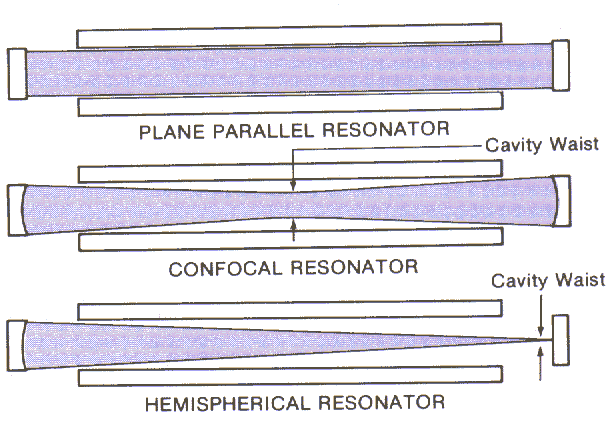
To sustain laser action it is usually necessary to place
the lasing material between the two mirrors of an
optical cavity. Some possible cavity designs are
shown below. Photons are reflected back and forth through the
lasing medium, which greatly increases the probability of stimulated emission.
The Helium- Neon (He-Ne) Laser
 For many years the most
common continuous wave (CW) laser was the He-Ne laser. These low-power (mW)
lasers use an electric discharge to create the population inversion. Most
He-Ne laser emit in the red at 633 nm. They are used in interferometers,
bar-code reader, and laser printers. They also are used for many sighting
and pointing applications from medical surgery to high-energy physics.
For many years the most
common continuous wave (CW) laser was the He-Ne laser. These low-power (mW)
lasers use an electric discharge to create the population inversion. Most
He-Ne laser emit in the red at 633 nm. They are used in interferometers,
bar-code reader, and laser printers. They also are used for many sighting
and pointing applications from medical surgery to high-energy physics.
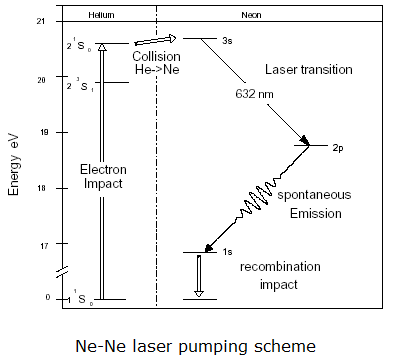
In a helium neon laser, a low pressure mixture of helium
and neon (<15%) is contained in a narrow bore glass tube. A
longitudinal, DC electrical discharge is maintained in the narrow tube.
This creates a plasma. Collisions with free electrons in the
plasma excite the helium atoms and cause a significant number of them to
be trapped in the lowest-energy metastable states. Neon is a
larger, more complex atom, with 10 electrons, which are arranged in a 1s22s22p6
configuration in the ground state. Neon has many excited states,
and the ones concerned with laser action are shown in the energy level
diagram on the right.
An excited state of neon occurs at excitation energies
similar to that of the lowest-energy metastable state of helium.
Collisions between excited state helium atoms and ground state neon
atoms cause some of the neon atoms to be excited to this state.
The 632.8 nm red laser output is
produced by the 3s - 2p transition in neon. (See diagram.) The energy level
labeled 2p lies ~18.6 eV above the ground state and therefore is
negligibly populated, even in the plasma. Consequently, a
population inversion between 3s and 2p is easily maintained by
collisional pumping with excited helium atoms. The states labeled
2p is populated by the laser transitions but is
rapidly depopulated by spontaneous emission to lower levels.
However, the state labeled 1s is metastable and must be depopulated by
collisions with the walls of the tube. That is why He-Ne lasers all
have very narrow-bore tubes.
 The helium neon laser is low gain device and require cavity mirrors of high
reflectance in order to achieve lasing action. Typically, one end of the
cavity is defined by a total reflector (reflectance >99.9%), and the
other end is defined by a mirror which permits ~ 1% of the intra cavity
light to escape, forming the useful output of the laser. It therefore
follows that the intra cavity beam is up to 200 times more intense than
the output beam.
The helium neon laser is low gain device and require cavity mirrors of high
reflectance in order to achieve lasing action. Typically, one end of the
cavity is defined by a total reflector (reflectance >99.9%), and the
other end is defined by a mirror which permits ~ 1% of the intra cavity
light to escape, forming the useful output of the laser. It therefore
follows that the intra cavity beam is up to 200 times more intense than
the output beam.
Problem:
A helium-neon laser emits laser light at a wavelength of 632.8 nm and a power
of 2.3 mW. At what rate are photons emitted by this device?
Solution:
- Reasoning:
Power = energy/second = 2.3*10-3 J/s.
Photon energy = hf = hc/λ.
- Details of the calculation:
Number of photons/s = power/photon energy = 7.3*1015/s.
Embedded Question 2
- Assume that in a laser material all the conditions for stimulated
emission are satisfied, and that a good pumping mechanism can establish a
population inversion. Why do you still need a cavity to make a laser?
Discuss this with your fellow students in the discussion forum!
 An atom or molecule can emit a photon via
spontaneous emission or
stimulated emission. Atoms and
molecules in excited states randomly emit single photons in all
directions according to statistical rules via spontaneous emission. In
the process of stimulated emission, a photon of energy hf perturbs an
excited atom or molecule and causes it to relax to a lower level,
emitting a photon of the same frequency, phase, and polarization as the
perturbing photon. Stimulated emission is the basis for photon
amplification and it is the fundamental mechanism underlying all laser
action.
An atom or molecule can emit a photon via
spontaneous emission or
stimulated emission. Atoms and
molecules in excited states randomly emit single photons in all
directions according to statistical rules via spontaneous emission. In
the process of stimulated emission, a photon of energy hf perturbs an
excited atom or molecule and causes it to relax to a lower level,
emitting a photon of the same frequency, phase, and polarization as the
perturbing photon. Stimulated emission is the basis for photon
amplification and it is the fundamental mechanism underlying all laser
action. Consider the simple case of a two-level system with
lower level 1 and upper level 2. Let N1 be the number
density of atoms in level 1 and N2 be the number density in
level 2.
Consider the simple case of a two-level system with
lower level 1 and upper level 2. Let N1 be the number
density of atoms in level 1 and N2 be the number density in
level 2.  There are several ways of
pumping a system to produce a
population inversion. It is, however, impossible to optically pump a
two-level system, since once N2 = N1 the induced
transition rate equals the absorption rate. Three or four levels are
needed to pump the system and produce the population inversion required
for laser action. Let us investigate the pumping of a four-level system.
There are several ways of
pumping a system to produce a
population inversion. It is, however, impossible to optically pump a
two-level system, since once N2 = N1 the induced
transition rate equals the absorption rate. Three or four levels are
needed to pump the system and produce the population inversion required
for laser action. Let us investigate the pumping of a four-level system.
 For many years the most
common continuous wave (CW) laser was the He-Ne laser. These low-power (mW)
lasers use an electric discharge to create the population inversion. Most
He-Ne laser emit in the red at 633 nm. They are used in interferometers,
bar-code reader, and laser printers. They also are used for many sighting
and pointing applications from medical surgery to high-energy physics.
For many years the most
common continuous wave (CW) laser was the He-Ne laser. These low-power (mW)
lasers use an electric discharge to create the population inversion. Most
He-Ne laser emit in the red at 633 nm. They are used in interferometers,
bar-code reader, and laser printers. They also are used for many sighting
and pointing applications from medical surgery to high-energy physics. The helium neon laser is low gain device and require cavity mirrors of high
reflectance in order to achieve lasing action. Typically, one end of the
cavity is defined by a total reflector (reflectance >99.9%), and the
other end is defined by a mirror which permits ~ 1% of the intra cavity
light to escape, forming the useful output of the laser. It therefore
follows that the intra cavity beam is up to 200 times more intense than
the output beam.
The helium neon laser is low gain device and require cavity mirrors of high
reflectance in order to achieve lasing action. Typically, one end of the
cavity is defined by a total reflector (reflectance >99.9%), and the
other end is defined by a mirror which permits ~ 1% of the intra cavity
light to escape, forming the useful output of the laser. It therefore
follows that the intra cavity beam is up to 200 times more intense than
the output beam.