To study multi-electron atoms, imagine that we start with hydrogen and add
electrons, one at a time. At the same time we increase the number of protons and
neutrons in the nucleus to keep the atom electrically neutral and the nucleus
stable. We will assume that as we add each electron, it falls down to the lowest
energy state available. If we start with a nucleus with one proton, and
drop in one electron, the electron eventually falls down to the n = 1 state. Its
energy is E1 = -13.6 eV. As we add a proton (and two neutrons) to
form a helium nucleus and drop in another electron, we can expect the electron
to also fall down to the n = 1 state. The extra Coulomb attractive force of the
two protons in the nucleus strengthens the binding of the electrons, but the
repulsive force between the two electrons weakens it. Experimentally, it takes
24.6 eV to remove an electron from helium, while only 13.6 eV are needed for
hydrogen. Thus the electrons are more tightly bound in helium, and we see that in helium the extra Coulomb attraction to the nucleus is more important than the repulsion
between electrons.
Using helium as a guide, we should expect that when we go to lithium with 3
protons in the nucleus, the increased Coulomb attraction to the nucleus should
cause lithium's three electrons to be even more tightly bound than helium's two. This would lead us to predict that it takes even more than 24.6 eV to pull one
of the electrons out of lithium.
This is not a good prediction. Experimentally, the amounts of energy needed
to remove electrons from lithium one at a time are 5.39 eV, 75.26 eV and 121.8
eV. While two of lithium's electrons are tightly bound, one is very loosely
bound, requiring less than half the energy to remove than it takes to remove the
electron from hydrogen. A possible explanation for the loose binding of
lithium's third electron is that, for some reason, the electron does not fall
down to the lowest energy n = 1 state. It appears to be hung up in one of the
higher energy, less tightly bound n = 2 states. For n = 2, there are 4 different
sets of quantum numbers (l, m) and 4 different wave functions.
Pauli Exclusion Principle
But why would the third lithium electron not fall down to the low energy n =
1 state? In 1925, two separate ideas provided the explanation. Wolfgang Pauli
proposed that no two electrons were allowed to be in exactly the same state. This is known as the
Pauli exclusion principle. But the exclusion principle seems to go too far, because in helium, both
electrons are in the same n = 1, l = 0, m = 0 state. If you cannot have two
electrons in exactly the same state in an atom, then something must be different
about the two electrons in helium.
Electron Spin
To explain this difference between the two electrons, two graduate students,
Samuel Goudsmit and George Uhlenbeck, proposed that the electron had its own
internal angular momentum. This became known as spin angular momentum. The
special feature of the electron's spin is that it has two allowed projections
along any axis, which we call spin up and spin down. In helium you could have
two electrons in the same n = 1 state if they had different spin projections,
because then they would not be in identical states. A state would be
characterized by 4 quantum numbers, n, l, m, and ms, where ms
tells us about the projection of the electron's spin on the z-axis. Because the
electron spin has only two allowed projections along any axis, we cannot add a
third electron to the n = 1 state. Lithium's third electron must stop at one of
the higher energy n = 2 states. Its energy is much less negative and therefore
this electron is much less tightly bound than the first two electrons that went
down to the n = 1 state.
The spin of a particle is closely related to its properties in statistical
mechanics. Particles with half-integer spin obey "Fermi-Dirac statistics" and are
known as fermions. They are subject to the
Pauli exclusion principle, which forbids them from sharing a quantum states. Particles with
integer spin, on the other hand, obey "Bose-Einstein statistics" and are known as
bosons. These particles can share
quantum states.
The periodic table
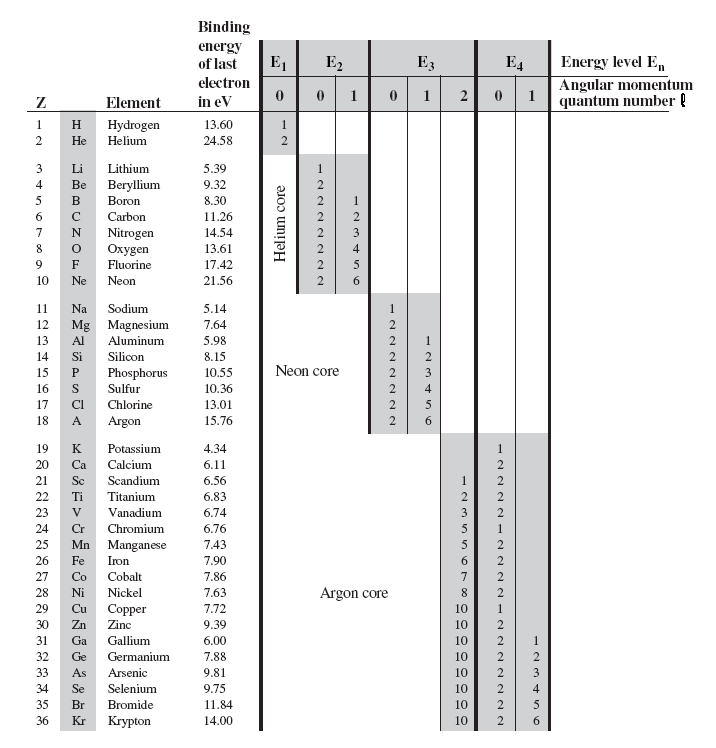 As we go to larger atoms,
adding electrons one at a time, the n = 2 states begin to fill up. Since
there are four different values for (l, m), each with two allowed spin
states, up to 8 electrons can fill the n = 2 states. All the states with
quantum number n = 2 are said to be in the n = 2 shell. A shell refers to all the states
with the same quantum number n. A subshell
refers to all the state with the same quantum numbers n and l. When the
n = 2 states are all filled we get to the element neon with two n = 1
electrons and eight n = 2 electrons. Neon is an inert noble gas that is
chemically similar to helium. Adding one more electron by going to
sodium, the eleventh electron has to go up into the n = 3 energy level
since both the n = 1 and n = 2 states are full. This eleventh electron
in sodium is loosely bound like the third electron in lithium, with the
result that both lithium and sodium have similar chemical properties. They are both strongly reactive metals. The table
on the right shows the
electron structure and the binding energy of the last (most loosely
bound) electron for the first 36 elements in the periodic table.
As we go to larger atoms,
adding electrons one at a time, the n = 2 states begin to fill up. Since
there are four different values for (l, m), each with two allowed spin
states, up to 8 electrons can fill the n = 2 states. All the states with
quantum number n = 2 are said to be in the n = 2 shell. A shell refers to all the states
with the same quantum number n. A subshell
refers to all the state with the same quantum numbers n and l. When the
n = 2 states are all filled we get to the element neon with two n = 1
electrons and eight n = 2 electrons. Neon is an inert noble gas that is
chemically similar to helium. Adding one more electron by going to
sodium, the eleventh electron has to go up into the n = 3 energy level
since both the n = 1 and n = 2 states are full. This eleventh electron
in sodium is loosely bound like the third electron in lithium, with the
result that both lithium and sodium have similar chemical properties. They are both strongly reactive metals. The table
on the right shows the
electron structure and the binding energy of the last (most loosely
bound) electron for the first 36 elements in the periodic table.
The general features of this table are that the lowest energy levels
fill up first, and there is a large drop in binding energy when we start
filling a new energy level. We see these drops when we go from the inert
gases helium, neon, and argon to the reactive metals lithium, sodium and
potassium. We can see that this sudden change in binding energy leads to
a significant change in the chemical properties of the atom. A closer
look at the table shows that there is a relatively steady uniform
increase in the electron binding as the energy level fills up. The
binding energy goes from 5.39 eV for lithium in fairly equal steps up to
21.56 eV for neon as the n = 2 energy level fills. The pattern is more
or less repeated as we go from 5.14 eV for sodium up to 15.76 eV for
argon while filling the n = 3 energy level. It repeats again in going
from 4.34 eV for potassium up to the 14.00 eV for krypton.
A closer look also uncovers some exceptions to the rule that the
lower n-states fill first. The most notable exception is at potassium,
where the n = 4 state with l = 0 begin to fill before the n = 3 states
with l = 2. To understand why the binding energy gradually
increases as a shell fills up, and why the n = 3 , l = 2 states fill up
late, we have to take a closer look at the electron wave functions and
see how their shape affects the binding energy.
 Electrons with no orbital angular momentum and
therefore quantum number l = 0, have a finite probability of being very
close to and even inside the nucleus. They therefore sometimes
feel a very strong, attractive force and are bound stronger to the atom.
Electrons with no orbital angular momentum and
therefore quantum number l = 0, have a finite probability of being very
close to and even inside the nucleus. They therefore sometimes
feel a very strong, attractive force and are bound stronger to the atom.
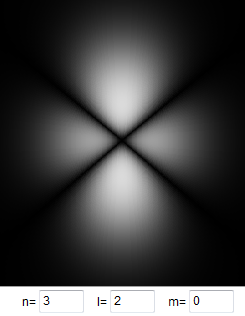 Electrons with non-zero orbital
angular momentum and therefore non-zero quantum number l, are never
found inside the nucleus. The larger l, the farther away they have
to stay from the nucleus. Since their potential energy U(r) is
proportional to -1/r, they never see the most negative regions of the
potential well and therefore are not as strongly bound to the atom.
Electrons with non-zero orbital
angular momentum and therefore non-zero quantum number l, are never
found inside the nucleus. The larger l, the farther away they have
to stay from the nucleus. Since their potential energy U(r) is
proportional to -1/r, they never see the most negative regions of the
potential well and therefore are not as strongly bound to the atom.
As n increases, the difference between energy levels with quantum
number n and n + 1 becomes smaller, and, starting with n = 3, electrons
are more strongly bound in the n + 1, l = 0 energy level than in the n, l
= 2 energy level. For example, electrons fill the n = 4, l = 0 subshell before they fill the n = 3, l = 2 subshell. Once the n =
4, l = 0 subshell has been filled, electrons can no longer have zero
angular momentum, and they resume filling the n = 3, l = 2 subshell.
Notation
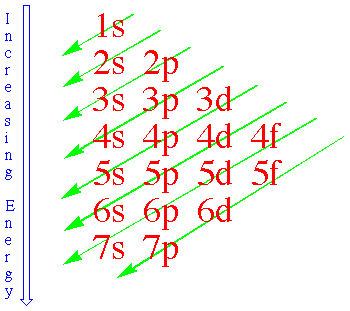 An electron configuration is an enumeration of the values of n and l for all electrons of an atom. The
standard notation to describe atomic electron configurations is to
denote each subshell by a number equal to its the principal quantum
number n, a letter referring to its angular momentum quantum number l
and a superscript giving the number of electrons in the subshell. The electron subshells of atoms in their ground state are filled one
electron at a time by putting each electron into the state with the
lowest available energy. The energy ordering of the subshells can be
remembered from the diagram on the right.
An electron configuration is an enumeration of the values of n and l for all electrons of an atom. The
standard notation to describe atomic electron configurations is to
denote each subshell by a number equal to its the principal quantum
number n, a letter referring to its angular momentum quantum number l
and a superscript giving the number of electrons in the subshell. The electron subshells of atoms in their ground state are filled one
electron at a time by putting each electron into the state with the
lowest available energy. The energy ordering of the subshells can be
remembered from the diagram on the right.
Examples:
| Element |
# of Electrons in Element |
Electron Configuration |
|
He |
2 |
1s2 |
|
Li |
3 |
1s22s1 |
|
Be |
4 |
1s22s2 |
|
O |
8 |
1s22s22p4 |
|
Cl |
17 |
1s22s22p63s23p5 |
|
K |
19 |
1s22s22p63s23p64s1 |
Electrons in a subshell with l = 0 have wave functions and probability
densities that are spherically symmetric. The probability density of an
electron in a subshell with l > 0 varies with angle. However, when a subshell is completely filled, then the probability of finding an
electron a given distance from the nucleus is the same for any angle.
The superposition of all the possible angular probability distributions
becomes again spherically symmetric.
Electrons are point particles. We either find an electron at a given
position, or we do not find it. If we find an electron, it has charge -qe. Only if we average the amount of charge found in a given volume
∆V over time can we define a charge
density or charge distribution ∆Q/∆V. This average charge density is proportional to the probability density. For a closed subshell the electron charge distribution is
spherically symmetric.
Problem:
How many subshells and electron states are in the n = 5 shell?
Solution:
- Reasoning:
Each subshell is characterized by a value of n and l. For n = 5 there are
5 possible values of l. We have 5 subshells.
- Details of the calculation:
l = 0: m = 0, ms =½, -½. There are 2 possible electron
states.
l = 1: 3 m values are possible, 2 ms values are possible. There
are 3*2 = 6 possible states.
l = 2: 5 m values are possible, 2 ms values are possible. There
are 5*2 = 10 possible states.
l = 3: 7 m values are possible, 2 ms values are possible. There
are 7*2 = 14 possible states.
l = 4: 9 m values are possible, 2 ms values are possible. There
are 9*2 = 18 possible states.
Total number of possible states: 2 + 6 + 10 + 14 + 18 = 50.
Problem:
(a) If one subshell of an atom has 9 electrons in it, what is the minimum
value of l?
(b) What is the spectroscopic notation for this atom, if this subshell is part
of the n = 3 shell and the atom is in its ground state.
Solution:
- Reasoning:
For a given l, there are 2l + 1 different values of m. For each m
there are two different values of ms.
So a subshell with quantum number l can hold 2(2l + 1) = 4l + 2 electrons.
- Details of the calculation:
(a) To hold 9 electrons the minimum value for l is 2.
(b) The electron subshells of atoms in their ground state are filled
one electron at a time by putting each electron into the state with the
lowest available energy.
The spectroscopic notation for the atom therefore is 1s22s22p63s23p64s23d9.
Problem:
In an atom, how many electron states are in the n = 3 shell?
Solution:
- Reasoning:
n = 3, l = 0, 1 , 2.
For l = 0, m = 0.
For l = 1, m = -1, 0, 1.
For l = 2, m = -2, -1, 0, 1, 2.
So we have 9 different (n, l, m) combinations.
Each of those can be associated with ms = -½ and ms
= ½.
We have 18 unique combinations of quantum numbers and can accommodate 18 electrons.
 As we go to larger atoms,
adding electrons one at a time, the n = 2 states begin to fill up. Since
there are four different values for (l, m), each with two allowed spin
states, up to 8 electrons can fill the n = 2 states. All the states with
quantum number n = 2 are said to be in the n = 2 shell. A shell refers to all the states
with the same quantum number n. A subshell
refers to all the state with the same quantum numbers n and l. When the
n = 2 states are all filled we get to the element neon with two n = 1
electrons and eight n = 2 electrons. Neon is an inert noble gas that is
chemically similar to helium. Adding one more electron by going to
sodium, the eleventh electron has to go up into the n = 3 energy level
since both the n = 1 and n = 2 states are full. This eleventh electron
in sodium is loosely bound like the third electron in lithium, with the
result that both lithium and sodium have similar chemical properties. They are both strongly reactive metals. The table
on the right shows the
electron structure and the binding energy of the last (most loosely
bound) electron for the first 36 elements in the periodic table.
As we go to larger atoms,
adding electrons one at a time, the n = 2 states begin to fill up. Since
there are four different values for (l, m), each with two allowed spin
states, up to 8 electrons can fill the n = 2 states. All the states with
quantum number n = 2 are said to be in the n = 2 shell. A shell refers to all the states
with the same quantum number n. A subshell
refers to all the state with the same quantum numbers n and l. When the
n = 2 states are all filled we get to the element neon with two n = 1
electrons and eight n = 2 electrons. Neon is an inert noble gas that is
chemically similar to helium. Adding one more electron by going to
sodium, the eleventh electron has to go up into the n = 3 energy level
since both the n = 1 and n = 2 states are full. This eleventh electron
in sodium is loosely bound like the third electron in lithium, with the
result that both lithium and sodium have similar chemical properties. They are both strongly reactive metals. The table
on the right shows the
electron structure and the binding energy of the last (most loosely
bound) electron for the first 36 elements in the periodic table. Electrons with no orbital angular momentum and
therefore quantum number l = 0, have a finite probability of being very
close to and even inside the nucleus. They therefore sometimes
feel a very strong, attractive force and are bound stronger to the atom.
Electrons with no orbital angular momentum and
therefore quantum number l = 0, have a finite probability of being very
close to and even inside the nucleus. They therefore sometimes
feel a very strong, attractive force and are bound stronger to the atom. Electrons with non-zero orbital
angular momentum and therefore non-zero quantum number l, are never
found inside the nucleus. The larger l, the farther away they have
to stay from the nucleus. Since their potential energy U(r) is
proportional to -1/r, they never see the most negative regions of the
potential well and therefore are not as strongly bound to the atom.
Electrons with non-zero orbital
angular momentum and therefore non-zero quantum number l, are never
found inside the nucleus. The larger l, the farther away they have
to stay from the nucleus. Since their potential energy U(r) is
proportional to -1/r, they never see the most negative regions of the
potential well and therefore are not as strongly bound to the atom. An electron configuration is an enumeration of the values of n and l for all electrons of an atom. The
standard notation to describe atomic electron configurations is to
denote each subshell by a number equal to its the principal quantum
number n, a letter referring to its angular momentum quantum number l
and a superscript giving the number of electrons in the subshell. The electron subshells of atoms in their ground state are filled one
electron at a time by putting each electron into the state with the
lowest available energy. The energy ordering of the subshells can be
remembered from the diagram on the right.
An electron configuration is an enumeration of the values of n and l for all electrons of an atom. The
standard notation to describe atomic electron configurations is to
denote each subshell by a number equal to its the principal quantum
number n, a letter referring to its angular momentum quantum number l
and a superscript giving the number of electrons in the subshell. The electron subshells of atoms in their ground state are filled one
electron at a time by putting each electron into the state with the
lowest available energy. The energy ordering of the subshells can be
remembered from the diagram on the right.