In addition to orbital angular momentum, many elementary particles have spin. Spin is intrinsic angular momentum
associated with elementary particles. It is a purely quantum-mechanical
phenomenon without any analog in classical physics. Spin is not associated
with any rotating internal parts of elementary particles; it is intrinsic to the
particle itself. An electron has spin, even though it is believed to be a
point particle, possessing no internal structure. Even a photon has spin.
 Like all angular momentum,
spin is quantized, and can only take on discrete values. The square of the magnitude of the
electron's spin is S2 = s(s + 1)ħ2 =
3/4ħ2. The spin quantum number s for the electron is
equal to ½. The spin angular momentum of an electron, measured along
any particular direction, can only take on the values ħ/2 or -ħ/2. We
have
Sz = msħ, with the quantum
number ms taking on values from -s to + s in integer steps, namely -½ and
+½. Electrons, protons, and neutrons are all "spin ½ particles".
Like all angular momentum,
spin is quantized, and can only take on discrete values. The square of the magnitude of the
electron's spin is S2 = s(s + 1)ħ2 =
3/4ħ2. The spin quantum number s for the electron is
equal to ½. The spin angular momentum of an electron, measured along
any particular direction, can only take on the values ħ/2 or -ħ/2. We
have
Sz = msħ, with the quantum
number ms taking on values from -s to + s in integer steps, namely -½ and
+½. Electrons, protons, and neutrons are all "spin ½ particles".
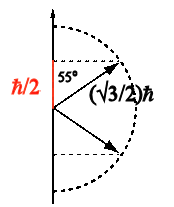
 We can measure the orientation of the electron spin
with respect to any axis (spin polarization). These
measurements yield two possible values, ~55o aligned or
anti-aligned. Measurements of the orientation with respect to
the x-, y-, and z-axis are incompatible.
We can measure the orientation of the electron spin
with respect to any axis (spin polarization). These
measurements yield two possible values, ~55o aligned or
anti-aligned. Measurements of the orientation with respect to
the x-, y-, and z-axis are incompatible.
Unlike orbital angular momentum, spin angular momentum is characterized by a
quantum number s that can be an integer or a half integer,
s = 0, ½, 1, 3/2, ... . The quantum number ms
specifying the alignment can take on values from -s to + s in integer steps.
There are elementary particles with integer and with half-integer spin.
The photon, for example, is a spin 1 particle, s = 1 for the photon. The
photon's spin is responsible for the light's macroscopic property of
polarization. To find the net spin of a composite particle (such as a
nucleus or an atom), we have to add
the spin vectors of its constituents. Composite particles can be integer
or half-integer spin particles.
 Like all angular momentum,
spin is quantized, and can only take on discrete values. The square of the magnitude of the
electron's spin is S2 = s(s + 1)ħ2 =
3/4ħ2. The spin quantum number s for the electron is
equal to ½. The spin angular momentum of an electron, measured along
any particular direction, can only take on the values ħ/2 or -ħ/2. We
have
Sz = msħ, with the quantum
number ms taking on values from -s to + s in integer steps, namely -½ and
+½. Electrons, protons, and neutrons are all "spin ½ particles".
Like all angular momentum,
spin is quantized, and can only take on discrete values. The square of the magnitude of the
electron's spin is S2 = s(s + 1)ħ2 =
3/4ħ2. The spin quantum number s for the electron is
equal to ½. The spin angular momentum of an electron, measured along
any particular direction, can only take on the values ħ/2 or -ħ/2. We
have
Sz = msħ, with the quantum
number ms taking on values from -s to + s in integer steps, namely -½ and
+½. Electrons, protons, and neutrons are all "spin ½ particles". We can measure the orientation of the electron spin
with respect to any axis (spin polarization). These
measurements yield two possible values, ~55o aligned or
anti-aligned. Measurements of the orientation with respect to
the x-, y-, and z-axis are incompatible.
We can measure the orientation of the electron spin
with respect to any axis (spin polarization). These
measurements yield two possible values, ~55o aligned or
anti-aligned. Measurements of the orientation with respect to
the x-, y-, and z-axis are incompatible.