 The mass analyzer:
The mass analyzer:Mass spectrometry has become an important measurement tool in clinical chemistry, microbiology, toxicology and in the pharmaceutical world. In order to measure the characteristics of individual molecules with a mass spectrometer, the molecules are ionized, accelerated, sorted according to their charge to mass ratio, and then detected. The three essential components of a mass spectrometer are:
Many ionization methods exist and the basic types are listed below.
| Basic type: | Name: | Ionizing Agent: |
|---|---|---|
| Gas Phase: | Electron Impact (EI) | Energetic electrons |
| Chemical Ionization (CI) | Gaseous ions | |
| Field Ionization (FI) | High-voltage electrode | |
| Desorption: | Field Desorption (FD) | High-voltage electrode |
| Electrospray Ionization (ESI) | High electric field | |
| Matrix Assisted Desorption/Ionization (MALDI) | Laser beam | |
| Plasma Desorption | Fission fragments from 252Cf | |
| Fast Atom Bombardment (FAB) | Energetic atomic beam | |
| Secondary Ion Mass Spectrometry (SIMS) | Energetic beam of ions | |
| Thermospray Ionization (TS) | High temperature |
Life scientists usually rely on two ionization methods: matrix-assisted laser desorption ionization (MALDI) and electrospray ionization (ESI). MALDI is a solid phase technique that can analyze a digested protein sample from a 2-D polyacrylamide gel. ESI, a liquid methodology, is compatible with high pressure liquid chromatography (HPLC) and capillary electrophoresis (CE).
 The mass analyzer:
The mass analyzer:Different types of mass analyzers exist. Here we
concentrate on the magnetic sector analyzer.
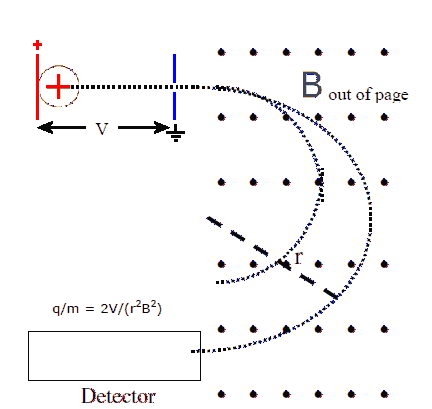
Ions are accelerated by passing them through a potential difference
V. A voltage is placed across parallel plates and ion are injected
at negligible speed into the area between the plates, near the plate
that holds charge of the same sign as the ions. The ions are
repelled from that plate and accelerate towards the other plate at
ground potential. A small hole in that plate lets them pass out of
the region between the plates.
When the ions enter the region between the plates, they have
negligible kinetic energy and potential energy
U = qV.
When the ions reach the ground plate, all the potential energy has been
converted into kinetic energy
Ekin = ½mv2 = qV.
The speed of the ions therefore is
v = (2qV/m)½.
The ions now enter the mass separation stage. This is simply a
region with a uniform magnetic field perpendicular to the velocity of the ions. The magnetic force F = qvB causes the ions
to move on a circular path with radius
r = mv/qB.
Inserting v from above we find the radius of this path
r = (m/q)½*(2V)½/B,
Which we can solve for the charge to mass ration
q/m = 2V/(r2B2).
In the separator shown, the ions are detected after they have
traveled half a circle in the mass separator. All the ions enter
the mass separator at the same point, but ions with different charge to
mass ratios travel on paths with different radii and exit at different
points.
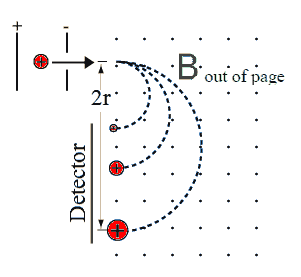 The detector
The detectorDetectors may be position sensitive, recording the arrival of ions as
well as their position on the detector, or they may be single channel
and not be able to record position information. A small slit is
then put in front of the detector and a scanning technique is used. The
detector can be placed at a fixed position and the magnitude of the B
field can be scanned, or the magnitude of B field can be fixed
and the detector position can be scanned.
All these techniques can produce graphs of the number of ions detected
versus the charge to mass ratio q/m. Biological molecules produce
several fragments when ionized. Mass spectrometry does not yield a
fragment's actual mass. Rather, it determines its mass-to-charge
ratio. If the ion charge is known, the mass can be calculated. The
fragments produce a mass spectrum that can be used to identify each molecule
uniquely.
The major barrier to productive mass spectrometry analysis is not the
instrumentation, but successful purification of bio-molecules suitable
for analysis.
That is why sample preparation has become the most critical and
challenging task in mass spectrometry analysis. It involves purifying, storing, and
recovering proteins, peptides, and other bio-molecules and removing such
contaminating species as buffers, salts, and detergents prior to MS
analysis.
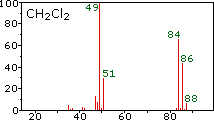
Example spectrum: