Consider a system in two different conditions, for example 1kg of ice at 0
oC, which melts and turns into 1 kg of water at 0 oC. We
associate with each condition a quantity called the entropy. The entropy of any substance is a function of the
condition of the substance. It is a physical property of the substance. For an ideal gas
Entropy is a function of its temperature and
volume, and for a solid and liquid it is a function of its temperature and
internal structure. The entropy is independent of the past history of the
substance. The entropy of the 1 kg of water at 0 oC is the same if
we obtained the water from ice, or if we cooled the water from room temperature
down to 0 oC. When a small amount of heat ΔQ is added to a substance
at temperature T, without changing its temperature appreciably, the entropy of
the substance changes by ΔS = ΔQ/T. When heat is removed, the entropy
decreases, when heat is added the entropy increases. Entropy has units of
Joules per Kelvin.
To calculate the change in entropy of a system for a finite process, when T
changes appreciably, we find some reversible path that can take the system
(infinitely slowly) from its initial
to its final state and evaluate ΔS for that path. The actual path
of the system from the initial to the final state may be very different and not
reversible. But the change in entropy depends only on the initial and final
state, not on the path.
An ideal frictionless reversible engine removes ΔQ1 from some
substance at T1, does some work, and delivers ΔQ2 at to
some other substance at T2, with ΔQ1/T1 = ΔQ2/T2.
The entropy of the substance at T1 decreases by ΔS1 = ΔQ1/T1
and the entropy of the substance at T2 increases by ΔS2 =
ΔQ2/T2, i.e. by the same amount. There is no net change in
entropy, if we consider the entire system. But a real engine always delivers
more heat at T2 than a reversible engine. For a real engine ΔS2
= ΔQ2/T2 is always greater than ΔS1 = ΔQ1/T1.
The entropy of the substance at T1 decreases, but the entropy of the
substance at T2 increases by a larger amount. The entropy of the
whole system increases.
The total entropy of a closed system is always increasing is
another way of stating the second law of thermodynamics.
A closed system is a system that does not interact in any way with its
surroundings. In practice there are really no closed systems except, perhaps,
the universe as a whole. Therefore we state the second law in the following
way: The total entropy of the universe is always
increasing. When entropy increases, a certain amount of energy becomes
permanently unavailable to do useful work.
Are the laws of physics reversible?
Evidently not! Where does this irreversibility come from? If
you videotape a sequence of events, and you run the tape backwards, it usually
does not take a very long time before everybody notices that something is
wrong. But when you look on a microscopic scale at any particular interaction,
such as a collision between two small particles, you find that no interaction
violates Newton's laws. On a microscopic scale each interaction is reversible.
Where then does the large-scale irreversibility come from?
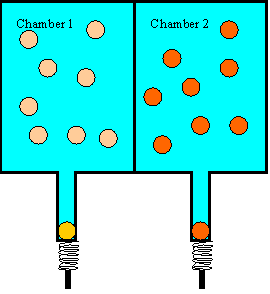 Let us look at a simple example of an irreversible process that is completely
composed of reversible events. Consider two chambers, separated by a dividing
wall. Assume we shoot 25 balls into chamber 1, each with 5 J kinetic energy.
The balls will bounce around in the chamber and hit the wall and each other. If
the walls of the chamber are perfectly hard and the coefficient of restitution
of the balls is 1, then the average kinetic energy of the balls in chamber 1
will stay 5 J, even so some balls will gain and some will lose energy in the
collisions. Assume we shoot 25 balls into chamber 2, each with 15 J of kinetic
energy. The average kinetic energy of these balls will stay 15 J. So as long
as chamber 1 and chamber 2 are separated by a dividing wall, the balls on one
side will be "hot" and the balls on the other side will be "cool". If we cut a
hole into the dividing wall, big enough for a ball to pass through, and wait
long enough, the average kinetic energy of the balls on either side of the wall
will be approximately 10 J. There will be 'hot" balls, with energies above 10
J, and "cool" balls, with energies below 10 J, on either side of the wall.
Let us look at a simple example of an irreversible process that is completely
composed of reversible events. Consider two chambers, separated by a dividing
wall. Assume we shoot 25 balls into chamber 1, each with 5 J kinetic energy.
The balls will bounce around in the chamber and hit the wall and each other. If
the walls of the chamber are perfectly hard and the coefficient of restitution
of the balls is 1, then the average kinetic energy of the balls in chamber 1
will stay 5 J, even so some balls will gain and some will lose energy in the
collisions. Assume we shoot 25 balls into chamber 2, each with 15 J of kinetic
energy. The average kinetic energy of these balls will stay 15 J. So as long
as chamber 1 and chamber 2 are separated by a dividing wall, the balls on one
side will be "hot" and the balls on the other side will be "cool". If we cut a
hole into the dividing wall, big enough for a ball to pass through, and wait
long enough, the average kinetic energy of the balls on either side of the wall
will be approximately 10 J. There will be 'hot" balls, with energies above 10
J, and "cool" balls, with energies below 10 J, on either side of the wall.
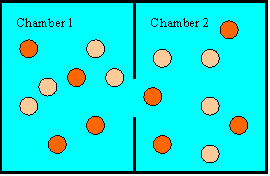 While Newton's laws do not forbid all the hot balls to gather on one side and
all the cool ones on the other side, the probability
that this will happen is practically zero. There are a very large number
of ways to distribute the energy among all the balls. Any one specific way is
equally likely or unlikely. It is just as unlikely for each ball to
have exactly 10 J of kinetic energy than for one ball to have 500 J and
all the others to have 0 J. But there are many more specific ways
of distributing the energy so that the average energy is approximately
equal on both sides than there are of distributing the energy so that
the average kinetic energy is three times as high on side 2 as it is on
side 1. There are many more ways of having a disorderly
arrangement than of having an orderly arrangement.&
While Newton's laws do not forbid all the hot balls to gather on one side and
all the cool ones on the other side, the probability
that this will happen is practically zero. There are a very large number
of ways to distribute the energy among all the balls. Any one specific way is
equally likely or unlikely. It is just as unlikely for each ball to
have exactly 10 J of kinetic energy than for one ball to have 500 J and
all the others to have 0 J. But there are many more specific ways
of distributing the energy so that the average energy is approximately
equal on both sides than there are of distributing the energy so that
the average kinetic energy is three times as high on side 2 as it is on
side 1. There are many more ways of having a disorderly
arrangement than of having an orderly arrangement.&
Irreversibility is a probabilistic notion. Events that do not
violate the laws of classical physics nevertheless do not
occur because they are just too improbable.
Disorder is more likely than order. But how do we quantify disorder?
The macrostate of a system is its state as viewed at a macroscopic level. For instance, to
describe the macrostate of an ideal gas in a cylinder, we could specify the
number of gas molecules N in the cylinder, the total volume V of the cylinder,
and total internal energy U of the gas molecules. As long as the gas is in
internal thermal equilibrium, these three parameters suffice to determine its macrostate. Other thermodynamic variables, such as the temperature T and the
pressure P of the gas, can then be calculated using the ideal gas law PV = NkT
and the relation U = (3/2)NkBT, where kB is the Boltzmann constant. In general,
the macrostate of a system is characterized by the numbers and types of
particles in the system and by internal parameters, such as mass, charge, and
spin, by external physical constraints on the system such as volume, electric
fields, and magnetic fields, and by conservation laws, such as energy
conservation.
The microstate of a system is its state as viewed at the molecular level. To describe the
microstate of the gas in the cylinder in classical mechanics, we have to specify
the exact position and velocity of each individual molecule. If we know a system's microstate, we also know its macrostate. On
the other hand, knowing a system's macrostate does not imply knowledge of its microstate.
The number of microstates that are consistent with a given macrostate is called
the multiplicity Ω of that macrostate. The multiplicity is the number of accessible
microstates, limited only by the constraints that determine the macrostate, i.e.
it is the number
of ways the insides of a system can be arranged so that from the outside things
looks the same. For our gas in the cylinder, the multiplicity is a function of
the three macroscopic variables N, V, and U. For most macrostates the
multiplicity is a very large number, since there are an enormous number of
different ways to distribute a given amount of energy among the system's N
molecules and to distribute the N molecules throughout the volume V.
The fundamental assumption of statistical mechanics is that
an isolated system in equilibrium in a given macrostate is
equally likely to be in any of its accessible microstates.
This means that the probability of finding the system in any given microstate
is 1/Ω, since there are Ω microstates, all equally probable. An isolated system
is a system of fixed composition, fixed external parameters and fixed total
energy. Equilibrium is the state in which the macroscopic properties are
independent of time.
The amount of disorder or the multiplicity Ω is the number of ways the insides
of a system can be arranged so that from the outside things looks the same.
It turns out that the logarithm of that number of ways is proportional the
entropy S.
S = kB lnΩ, where kB is the Boltzmann constant.
We can define the entropy as the logarithm of the disorder times a constant of
proportionality. When we change the entropy of a substance by an amount ΔS =
ΔQ/T, we change the disorder of the substance. Entropy always increases,
because a high amount of disorder is, by definition, is more likely than a low
amount of disorder. With our definition of disorder as the multiplicity of
the macrostate, every condition of a
system has a well-defined disorder. If this disorder is small, then in
common, everyday language, we say that the system is ordered.
We have two ways of figuring out if the entropy of a substance changes.
Both ways will lead to the same answer. Sometimes it is easier to get the
answer by considering the heat transfer to or from the substance. Sometimes it
is hard to follow the heat, but it is easy to decide if the disorder increases
or decreases.
Assume that during an irreversible process the entropy of a system increases
by ΔS. Then an amount of energy in the system, E = TminΔS, is no
longer available for useful work. Here Tmin is the lowest
temperature available to the system. Entropy is measure of energy quality.
Given two systems with identical energy content, the one with the lower entropy
contains the higher quality energy and can do more useful work.
Here are some situations in which entropy increases:
- The entropy increases whenever heat flows from a hot object to a cold
object. It increases when ice melts, water is heated, water boils, water
evaporates.
- The entropy increases when a gas flows from a container under high
pressure into a region of lower pressure. It increases when you spray something out of an aerosol can or you let
air out of a tire.
- The entropy increases whenever ordered energy is converted into
disordered energy. Kinetic friction and drag always increase entropy.
When water freezes its entropy decreases. This does not violate the second
law of thermodynamics. The second law does not say that entropy can never
decrease anywhere. It just says that the total entropy of the universe can
never decrease. Entropy can decrease somewhere, provided it increases somewhere
else by at least as much. The entropy of a system decreases only when it
interacts with some other system whose entropy increases in the process. That is the law.
Problem:
An ice tray contains 500 g of water. Calculate the change in entropy of
the water as it freezes completely and slowly at 0 oC.
Solution:
- Reasoning:
The water freezes at 0 oC = 273 K. ΔS = ΔQ/T
ΔQ = -mL, m = mass of water, L =
latent heat of fusion = 333000 J/kg.
- Details of the calculation:
ΔS = -(0.5 kg)(333000J /kg)/273 K = -610 J/K.
Problem:
If you toss two dice, what is the total number of ways that you can
obtain
(a) a 12 and
(b) a 7?
Solution:
- Reasoning:
(a) There is only one way to obtain a 12. Both dice must show a 6.
(b) There are six ways to obtain a 7. If you label the dice by A and
B, then there are six possible numbers you can throw with dice A, 1-6. Each
of these numbers has exactly one corresponding number that you must throw
with dice B, 6-1, to obtain 7.
Problem:
The surface of the Sun is approximately at 5700 K, and the temperature
of the Earth's surface is approximately 290 K. What entropy changes occur
when 1000 J of thermal energy is transferred from the Sun to the Earth?
Solution:
- Reasoning:
During the process the temperatures of the sun and the earth do not change
appreciably.
The change in the entropy of the sun is therefore ΔS = -1000 J/5700 K = -0.175 J/K.
The change in the entropy of the
earth is ΔS = 1000J/290 K = 3.448 J/K.
The entropy of the sun-earth
system increases by 3.27 J/K.
Entropy and life
The entropy of isolated
systems cannot decrease. However, when a system is not isolated, but is in
contact with its surrounding, then the entropy of this open system may decrease, with a necessary compensating increase in
the entropy of the surroundings.
Living systems are
characterized by their energy content and by the amount of energy flowing
through the system. Living systems are non-equilibrium systems. Energy flows
from a source through the living system into to a sink. The living system may
be well ordered since it is not an isolated system.
Energy enters into the
biosphere as radiation from the sun. It is stored as chemical energy. Biological cycles involve a series of biochemical reactions which are
accompanied by the production of thermal energy. Heat flows into the immediate
surroundings and finally as radiation into outer space. Three rules govern
every change.
-
The number of atoms is
conserved.
-
Energy is conserved
-
Entropy increases.
A living system is an
improbable non-equilibrium state. A state in thermodynamic equilibrium is a
very probable state, but it represents a dead system. To keep a system alive, it is
necessary to constantly do work to move the living system back into the
improbable state
it is drifting out of. For this to be possible, the system must be
connected to a source and a sink. The entropy of the source decreases, but the
entropy of the sink increases by a larger amount. The living organism can only
exist if the entropy of the rest of the universe is increasing.
 Let us look at a simple example of an irreversible process that is completely
composed of reversible events. Consider two chambers, separated by a dividing
wall. Assume we shoot 25 balls into chamber 1, each with 5 J kinetic energy.
The balls will bounce around in the chamber and hit the wall and each other. If
the walls of the chamber are perfectly hard and the coefficient of restitution
of the balls is 1, then the average kinetic energy of the balls in chamber 1
will stay 5 J, even so some balls will gain and some will lose energy in the
collisions. Assume we shoot 25 balls into chamber 2, each with 15 J of kinetic
energy. The average kinetic energy of these balls will stay 15 J. So as long
as chamber 1 and chamber 2 are separated by a dividing wall, the balls on one
side will be "hot" and the balls on the other side will be "cool". If we cut a
hole into the dividing wall, big enough for a ball to pass through, and wait
long enough, the average kinetic energy of the balls on either side of the wall
will be approximately 10 J. There will be 'hot" balls, with energies above 10
J, and "cool" balls, with energies below 10 J, on either side of the wall.
Let us look at a simple example of an irreversible process that is completely
composed of reversible events. Consider two chambers, separated by a dividing
wall. Assume we shoot 25 balls into chamber 1, each with 5 J kinetic energy.
The balls will bounce around in the chamber and hit the wall and each other. If
the walls of the chamber are perfectly hard and the coefficient of restitution
of the balls is 1, then the average kinetic energy of the balls in chamber 1
will stay 5 J, even so some balls will gain and some will lose energy in the
collisions. Assume we shoot 25 balls into chamber 2, each with 15 J of kinetic
energy. The average kinetic energy of these balls will stay 15 J. So as long
as chamber 1 and chamber 2 are separated by a dividing wall, the balls on one
side will be "hot" and the balls on the other side will be "cool". If we cut a
hole into the dividing wall, big enough for a ball to pass through, and wait
long enough, the average kinetic energy of the balls on either side of the wall
will be approximately 10 J. There will be 'hot" balls, with energies above 10
J, and "cool" balls, with energies below 10 J, on either side of the wall. While Newton's laws do not forbid all the hot balls to gather on one side and
all the cool ones on the other side, the probability
that this will happen is practically zero. There are a very large number
of ways to distribute the energy among all the balls. Any one specific way is
equally likely or unlikely. It is just as unlikely for each ball to
have exactly 10 J of kinetic energy than for one ball to have 500 J and
all the others to have 0 J. But there are many more specific ways
of distributing the energy so that the average energy is approximately
equal on both sides than there are of distributing the energy so that
the average kinetic energy is three times as high on side 2 as it is on
side 1. There are many more ways of having a disorderly
arrangement than of having an orderly arrangement.&
While Newton's laws do not forbid all the hot balls to gather on one side and
all the cool ones on the other side, the probability
that this will happen is practically zero. There are a very large number
of ways to distribute the energy among all the balls. Any one specific way is
equally likely or unlikely. It is just as unlikely for each ball to
have exactly 10 J of kinetic energy than for one ball to have 500 J and
all the others to have 0 J. But there are many more specific ways
of distributing the energy so that the average energy is approximately
equal on both sides than there are of distributing the energy so that
the average kinetic energy is three times as high on side 2 as it is on
side 1. There are many more ways of having a disorderly
arrangement than of having an orderly arrangement.&