The photoelectric effect
In the last lab you observed diffraction and interference. You
studied the diffraction and interference patters produced by single and
multiple slits and verified that one can predict the positions of the maxima and
minima in these patterns by assuming that light is an EM wave. Where
crests meet crests and troughs meet troughs we predict and observe maxima or
bright regions, and where crests meet troughs we predict and observe minima or
dark regions.
So light is a wave, these experiments settle
this? Not so fast!
In this lab you will simulate an experiment that suggests that light is
a particle. You will investigate the photoelectric effect. To eject an electron from a metal
surface a certain amount of energy Φ must be supplied to this electron. Φ
is called the work function of the metal. (If no energy
were required to free the electrons, they would just leave ordinary pieces of
metal.) In the wave picture the energy of the light beam does not depend
on the frequency, but only on the intensity, which is proportional to the square
of the amplitude. Einstein explained the photoelectric effect by
postulating that an electron can only receive the large amount of energy
necessary to escape the metal from the EM wave by absorbing a single photon. If this photon has enough energy,
the electron is freed. Excess energy appears as kinetic energy of the
electron. The maximum kinetic energy of the electron is given by E = hf -
Φ. If the photon does not have enough energy, then the electron cannot
escape the metal.
In this lab you will direct light with different wavelength onto a metal surface and measure the kinetic energy of the
photoelectrons ejected from the metal as a function of the frequency of the
light used to eject the electrons. You will measure the work function of
the metal and also determine the value of Planck's constant from your data.
This experiment reveals the "particle nature" of light. A second
experiment in the studio session will reveal the "wave nature" of electrons.
You will observe and analyze electron diffraction.
Open a Microsoft Word document to keep a log of your
experimental procedures, results and discussions. This log will
become your lab report. Address the
points highlighted in blue. Answer all questions.
Experiment 1
You will use an on-line simulation
from the University of Colorado PhET group.
Link to the simulation:
http://phet.colorado.edu/en/simulations/photoelectric
Explore the interface. There are some non-obvious controls.
- You can select Show photons in the Options menu to show the
light beam as composed of individual photons.
- You can select Control photon number instead of intensity in
the Options menu to change the Intensity slider to a Number of photons
slider.
- You can use the camera icon to take a snapshot of the graphs
so that you can compare graphs for different settings.
- You can Pause the simulation and then use Step to incrementally
analyze.
In the simulation photons strike a metal cathode and eject
electrons. Electrons are ejected with a range of energies, up to a maximum
energy. The electrons are collected on the anode and then flow back from
the anode to the cathode through a wire. The current in the wire is
measured. Electrons
are negatively charged. If the anode is at a positive voltage compared to
the cathode, electrons are attracted and gain energy. If the anode is at a
negative voltage compared to the cathode, electrons are repelled and loose
energy. Let Vca = Vcathode - Vanode.
If this potential difference Vca (energy per charge) becomes large
enough, electrons will no longer reach the anode and current will no longer flow
in the wire.
It is convenient to measure electron energy in units of electron volt (eV). In SI units 1 eV = 1.6*10-19 J.
If the potential difference between
the cathode and anode is x volts (Vca = x V), then electrons ejected from the
cathode need an energy of at least x eV to overcome this potential difference
and to reach the anode. By determining voltage Vca needed to reduce the
current in the wire to zero, we can determine the maximum energy
of the ejected electrons. That voltage is called the stopping voltage, Vstop
= Emax/qe = (hf - Φ)/qe.
The maximum energy E of electrons that reach the anode in eV has the same
numerical value as the voltage in Vstop.
(a) Exploration:
For a Sodium target discuss:
- For a fixed number of photons
and zero battery voltage, how does the number of
photoelectrons ejected depend on the wavelength? Does every photon eject an
electron? Does the probability of ejection change with wavelength?
Discuss!
- For a fixed wavelength and
zero battery voltage, how does the
current depend on the light intensity? Discuss!
- For a fixed wavelength and light intensity, how does
the current depend on the battery voltage?
- For a fixed wavelength and light intensity, do all
ejected electrons have the same energy? How can you measure the
maximum energy of the ejected electrons.
(b) Measurement:
Use a Sodium target. Set the
intensity to 100%. For the wavelengths listed in the table below, find the
maximum energy of the ejected electrons by finding the battery voltage Vca
that just prevents the most energetic electrons from reaching the anode.
Note: The text box under the battery displays -Vca.
| Wavelength (nm) |
Frequency (s-1) |
Maximum Electron Energy (eV) |
| 150 |
2.00e15 |
|
| 200 |
1.50e15 |
|
| 300 |
1.00e15 |
|
| 400 |
0.75e15 |
|
| 500 |
0.60e15 |
|
Prepare a graph of the maximum electron energy versus
the frequency of the light. Choose an X-Y Scatter plot. Plot maximum electron energy on the vertical axis and frequency on the
horizontal axis.
Prediction: E = hf - Φ
This equation is of the form y = ax + b, with a being the slope and b being the
y-intercept. The slope of the plot of E versus f will yield Planck's constant
and the y-intercept will yield the work function of the metal cathode.
Add a trendline and insert the equation for the trendline into your plot. Use
the slope of your trendline to find Planck's constant h and the intercept value
to find the work function Φ of Sodium. If
there are data points that seem to deviate to far from the trendline, repeat
those measurements until you are satisfied that you have done your best.
Since the electron energy is measured in eV and the
frequency in 1/seconds, Planck's constant h will have units of eV s and the work
function will have units of eV. Convert Planck's constant to SI units (J s) by
multiplying the value you obtained from the slope by 1.6*10-19J/eV.
- Copy the table into your Word document.
- What value did you obtain for h in units of eV s and J s?
- How does this value compare with the accepted value
h = 6.626*10−34 J s
= 4.136*10−15 eV s?
- Describe how the maximum energy of the photoelectrons
depends on the wavelength of the incident light.
- Defend whether this experiment supports a wave or a quantum
model of light based on your lab results.
Demo:
Link:
Photoelectric Effect - YouTube
Experiment 2
How does matter behave on a scale of a few nanometers or smaller? Is
its behavior governed by Newton's laws or by a wave equation? The de
Broglie relations associate a wavelength λ = h/p = h/√(2mE) with each particle
of momentum p. For an electron which has been accelerated through a
potential difference of 5 kV and therefore has a kinetic energy of 5000 eV =
8*10-16 J, this wavelength is λ = 1.74*10-11 m. Can
we measure λ?
If a beam of accelerated electrons passes through a thin crystal, the crystal
planes can act like a diffraction grating.
Different crystal planes can produce different diffraction patterns.
Planes that are spaced farther apart produce a narrower patterns. You will observe the diffraction pattern produced
when 5 keV electrons pass
through graphite.
For light normally incident on a grating with slit spacings d, we find
diffraction maxima at angles θ away from the normal such that dsinθ = mλ.
If we observe the diffraction pattern on a screen a distance L away from the
grating, then we can write
dz/(mL) = λ if θ is
a small angle. For electron diffraction from crystal planes at small
angles away from the forward direction, we can use the same formula dz/(mL) = λ to find the
diffraction maxima.
Graphite has the crystal structure shown below.
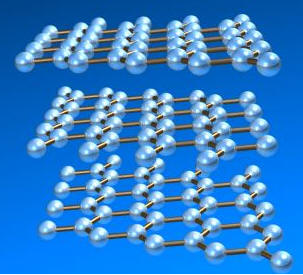
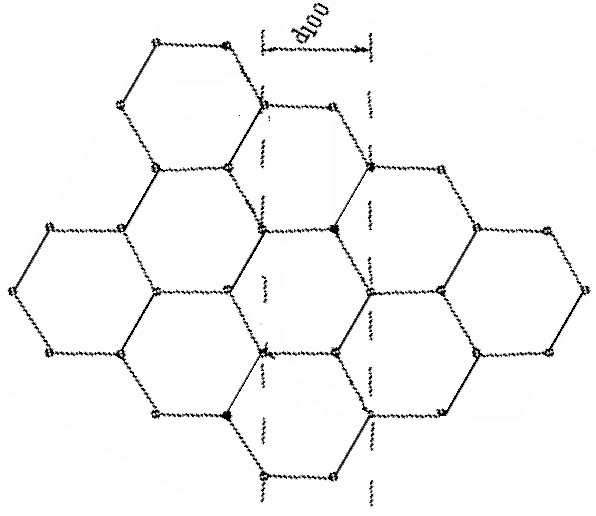
In this orientation, the d100 planes produce a horizontal pattern.
d100 = 2.10*10-10 m
 .
.
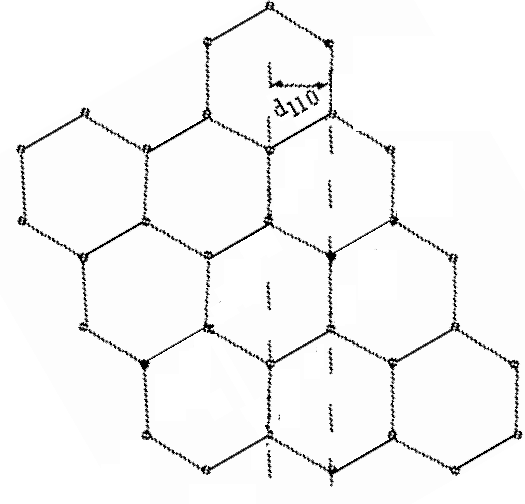
In this orientation, the d110 planes produce a horizontal pattern.
d110 = 1.21*10-10
m.
The graphite in our apparatus is not a single crystal, but a polycrystalline
powder. The orientation of the various crystals is random.
Powder diffraction produces a pattern of concentric rings. The powder
diffraction patters is just the superposition of the patterns produced by the
individual crystals with random orientations.
Assume for a single crystal with a fixed
orientation we observe the pattern below. The
diffraction pattern is rotated by the same angle as the crystal.
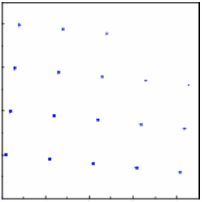
Then for 4 crystals with 4 different orientations,
we also observe 4 different orientations of the diffraction
patterns. The individual diffraction patterns plotted in the
same color as the corresponding crystal start to add up to rings.
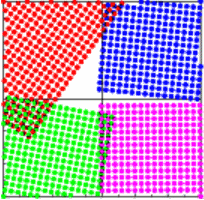
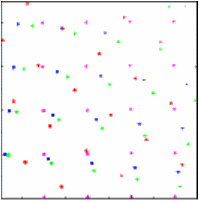
For 40 randomly oriented crystals,
powder rings become clearly visible.
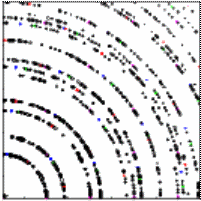
 In our experiment accelerated electron with 5 keV
kinetic energy pass through a graphite target in an evacuated tube and
hit a fluorescent screen. We observe the ring pattern on this
screen.
In our experiment accelerated electron with 5 keV
kinetic energy pass through a graphite target in an evacuated tube and
hit a fluorescent screen. We observe the ring pattern on this
screen.
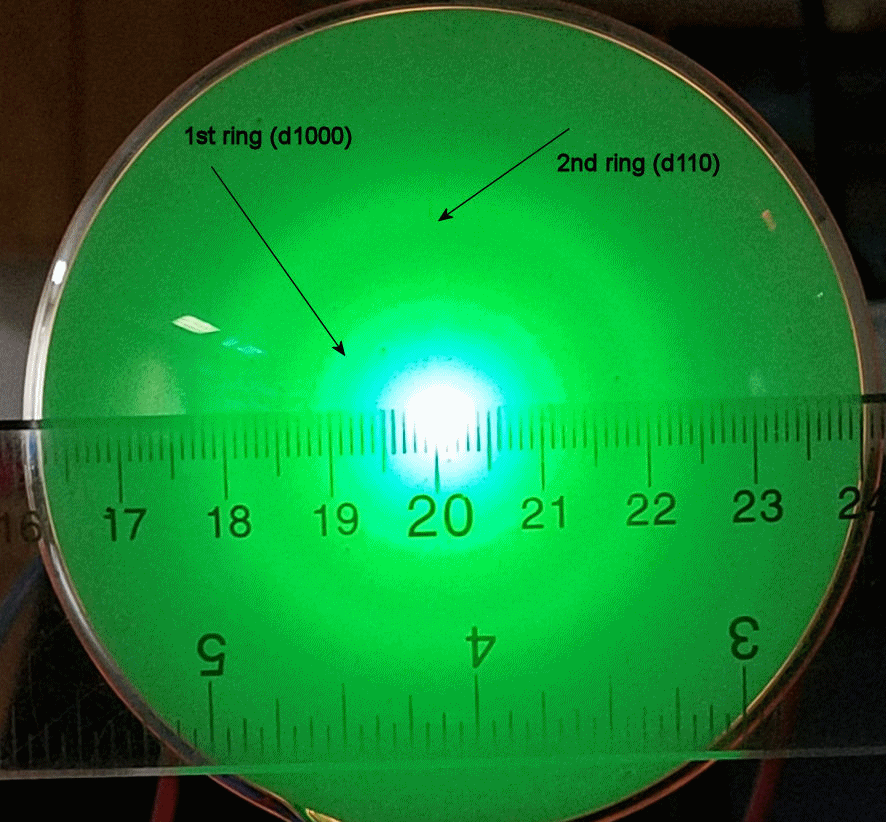
Two rings are clearly visible. These correspond to the
first-order maxima produced by the d100 and the d110 planes. All
other rings are too dim or at angles too small or too large to observe with our
apparatus.
You will measure the diameter of
the two visible rings and calculate the angles θ, given the distance L =
13.5 cm from the target to the screen.
Using the known plane spacings d100 = 2.10*10-10 m
and d110 = 1.21*10-10
m you will then use
dz/L = λ
to experimentally determine the de Broglie wavelength λ of the 5 keV electrons.
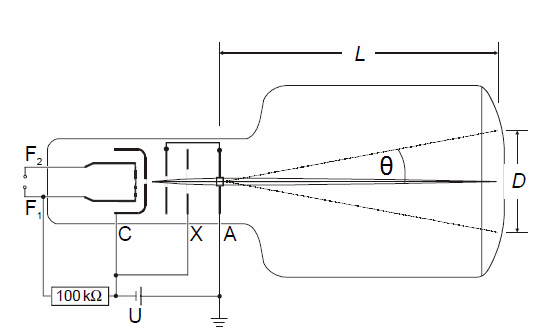
Schematic sketch off the apparatus:
L = 13.5 cm (distance between graphite foil and screen)
D = diameter of a diffraction ring observed on the screen
z = D/2
Demo:
Link:
Electron Diffraction Cathode Ray tube - YouTube
Link: Using an
electron diffraction tube - YouTube (longer clip)
Make the measurements and fill in the table below. Measure the center-to-center distance for each
bright ring. The scale on the picture is a mm scale.
| |
1st ring (d100) |
2nd ring (d110) |
| d (m) |
2.10E-10 |
1.21E-10 |
| L (m) |
0.135 |
0.135 |
| D (m) |
|
|
| z |
|
|
| λ = dz/L |
|
|
Discuss your results.
- Copy the table into your Word document.
- Do the two values for λ agree within
experimental uncertainty?
- What do you think contributes most to the
experimental uncertainty?
- Is your experimental value of λ close to the expected
de Broglie wavelength of the electrons?
- Does this experiment convince you that
electrons do not behave like classical particles, or can you think
of a classical explanation for your results?
Convert your log into a lab report.
See the grading scheme for all lab
reports.
Name:
E-mail address:
Laboratory 10 Report
- In one or two sentences, state the goal of this lab.
- Make sure you completed the entire lab and answered all parts. Make
sure you show your work and inserted and properly labeled relevant tables
and plots.
- Add a reflection at the end of your report in a short essay format.
Save your Word document (your name_lab10.docx), go to Canvas, Assignments, Lab
10 and submit your document.
 In our experiment accelerated electron with 5 keV
kinetic energy pass through a graphite target in an evacuated tube and
hit a fluorescent screen. We observe the ring pattern on this
screen.
In our experiment accelerated electron with 5 keV
kinetic energy pass through a graphite target in an evacuated tube and
hit a fluorescent screen. We observe the ring pattern on this
screen.

 .
.






