One of the earliest scientists to be intrigued by heat engines was a French engineer named Sadi Carnot (1796-1832). A heat engine uses heat transfer to do work in a cyclical process. After each cycle the engine returns to its original state and is ready to repeat the conversion process (disordered --> ordered energy) again.
Carnot postulated that heat cannot be taken in at a certain temperature with no other change in the system and converted into work. This is one way of stating the second law of thermodynamics.
Carnot assumed that an ideal engine, converting the maximum amount of thermal energy into ordered energy, would be a frictionless engine. It would also be a reversible engine. By itself, heat always flows from an object of higher temperature to an object with lower temperature. A reversible engine is an engine in which the heat transfer can change direction, if the temperature of one of the objects is changed by a tiny (infinitesimal) amount. When a reversible engine causes heat to flow into a system, it flows as the result of infinitesimally small temperature differences, or because there is an infinitesimal amount of work done on the system. If such a process could be actually realized, it would be characterized by a continuous state of equilibrium (i.e. no pressure or temperature differentials) and would occur at a rate so slow as to require an infinite time. The momentum of any component of a reversible engine never changes abruptly in an inelastic collision, since this would result in an irreversible, sudden increase of disordered energy of that component. A real engine always involves at least a small amount of irreversibility. Heat will not flow without a temperature differential and friction cannot be entirely eliminated.
Carnot showed that if an ideal reversible engine, called a Carnot engine, picks up the amount of heat Q1 from a reservoir at temperature T1, converts some of it into useful work, and delivers the amount of heat Q2 to a reservoir at temperature T2, then Q1/T1 = Q2/T2. Here T is the absolute temperature, measured in Kelvin and a heat reservoir is a system, such as a lake, that is so large that its temperature does not change when the heat involved in the process considered flows into or out of the reservoir. To convert heat into work, you need at least two places with different temperatures. If you take in Q1 at temperature T1 you must dump at least Q2 at temperature T2.
An example of an idealized, frictionless engine in which all the processes are reversible is an ideal gas in a cylinder equipped with a frictionless piston. The cylinder alternates in making contact with one of two heat reservoirs at temperatures T1 and T2 respectively, with T1 higher than T2.
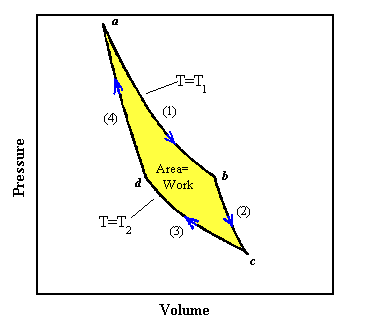
During one cycle we have put an amount of heat Q1 into the gas at temperature T1 and have removed an amount of heat Q2 at temperature T2. Using the relationships between ΔU, ΔQ, and ΔW for the different thermodynamic processes, we can show that Q1/T1 = Q2/T2.
Link: Mathematical details using Calculus
The useful work done by a heat engine is W = Q1 - Q2 (energy conservation). An ideal reversible engine does the maximum amount of work.
Any real engine delivers more heat Q2 at the reservoir at T2 than a reversible one and therefore does less useful work.
The maximum amount of work you can therefore get out of a heat engine is the amount you get from an ideal, reversible engine.
Wmax = Q1 - Q2 = Q1 - Q1 T2/T1 = Q1 (1 - T2/T1).
W is positive if T1 is greater than T2.
The efficiency of a heat engine is the ratio of the work obtained to the heat energy put in at the high temperature, e = W/Qhigh. The maximum possible efficiency emax of such an engine is
emax = Wmax/Qhigh = (1 - Tlow/Thigh) = (Thigh - Tlow)/Thigh.
Assume you have a reservoir of hot water at temperature T1. Can you take an amount of heat Q1 out of this reservoir and convert it into work? No! You can convert a fraction of the heat into work if you have a place at a lower temperature T2 where you can dump some of the heat. An engine that does work by removing heat from a reservoir at a single temperature cannot exist.
Heat cannot be taken in at a certain temperature with no other change in the system and converted into work. This is one way of stating the second law of thermodynamics.
Heat cannot, of itself, flow from a cold to a hot object is another way of stating the second law of thermodynamics.
If it could, then heat dumped at T2 could just flow back to the reservoir at T1 and the net effect would be an amount of heat ΔQ = Q1 - Q2 taken at a T1 and converted into heat with no other changes in the system.
A certain gasoline engine has an efficiency of 30.0%. What would the
hot reservoir temperature be for a Carnot engine having that efficiency, if it
operates with a cold reservoir temperature of 200 oC?
Solution:
An inventor is marketing a device and claims that it takes 25 kJ of heat at
600 K, transfers heat to the environment at 300 K, and does 12 kJ of work.
Should you invest in this device?
Solution:
Note:
Disordered energy cannot be completely converted back to ordered energy.
The maximum efficiency of a heat engine converting thermal to ordered energy is
100%*(Thigh - Tlow)/Thigh.
Here Thigh and Tlow are the highest and lowest temperature
accessible to the engine.
Ordered energy, on the other hand, can be completely converted into other forms of energy. The maximum efficiency of an engine using ordered energy is 100%.